Environment & Energy
Related: About this forumCatalytic Combo Converts CO2 to Solid Carbon Nanofibers
https://www.bnl.gov/newsroom/news.php?a=121635Tandem electrocatalytic-thermocatalytic conversion could help offset emissions of potent greenhouse gas by locking carbon away in a useful material
January 11, 2024
UPTON, NY—Scientists at the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory and Columbia University have developed a way to convert carbon dioxide (CO₂ ), a potent greenhouse gas, into carbon nanofibers, materials with a wide range of unique properties and many potential long-term uses. Their strategy uses tandem electrochemical and thermochemical reactions run at relatively low temperatures and ambient pressure. As the scientists describe in the journal Nature Catalysis, this approach could successfully lock carbon away in a useful solid form to offset or even achieve negative carbon emissions.
“You can put the carbon nanofibers into cement to strengthen the cement,” said Jingguang Chen, a professor of chemical engineering at Columbia with a joint appointment at Brookhaven Lab who led the research. “That would lock the carbon away in concrete for at least 50 years, potentially longer. By then, the world should be shifted to primarily renewable energy sources that don’t emit carbon.”
As a bonus, the process also produces hydrogen gas (H₂ ), a promising alternative fuel that, when used, creates zero emissions.
Capturing or converting carbon
The idea of capturing CO₂ or converting it to other materials to combat climate change is not new. But simply storing CO₂ gas can lead to leaks. And many CO₂ conversions produce carbon-based chemicals or fuels that are used right away, which releases CO₂ right back into the atmosphere.
…
So, to reverse things on a grand scale, say, convert 120ppm of CO₂ to carbon nanofibers would require, let’s call it 3 times the amount of useful energy that we produced by burning it in the first place. On the other hand, pumping CO₂ into basalt does not have to pay back that chemical energy debt.
thatdemguy
(522 posts)Where does the hydrogen come from? C02 minus the C is oxygen, I still dont know where the hydrogen comes from.
Never mind I just read it, they have to add water.
keithbvadu2
(40,092 posts)Nano particles of plastic are becoming more pervasive in our environment and our bodies.
Even in large blocks, the carbon nanofibers would eventually become nano particles again but maybe we could slow down the effects.
Or is it no worries?
OKIsItJustMe
(20,735 posts)For the last million years or so, atmospheric CO₂ has not risen above about 300ppm. When it has, it has been associated with about 5°C of warming. (We would prefer to limit it to no more than 1.5°C.)

Atmospheric CO₂ levels are at about 420ppm and rising over 2ppm each year.
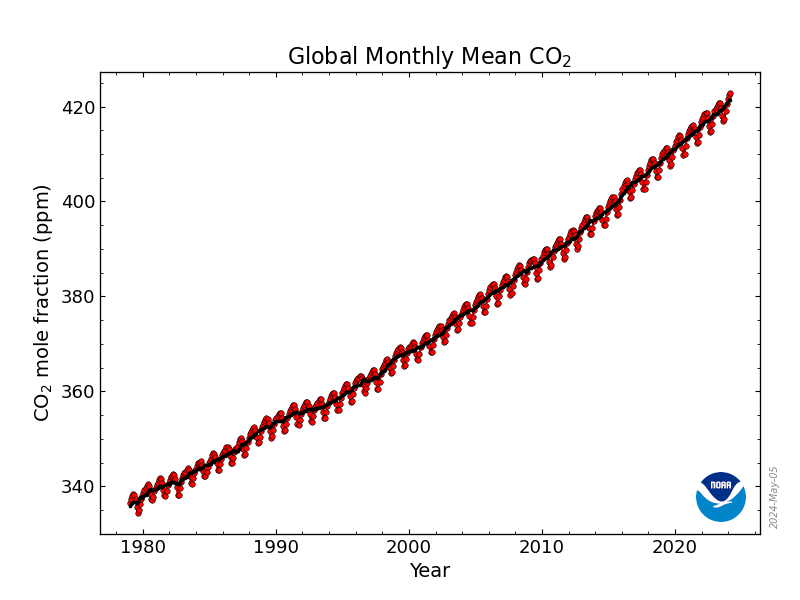
There are various schemes about how we can decrease the rate of emissions, but I haven’t seen any plans to decrease the accumulation which I consider practical.
To give you an idea of the magnitude of the challenge, before we got involved, burning fossil fuels, cutting down forests and burning the wood, digging up peat and burning it (you get the idea) a pristine ecosystem was able to draw down CO₂ about 1ppm in about 1,000 years.
We’d probably do well to decrease CO₂ levels like… 150ppm? (In 2008, James Hansen et. al suggested using 350ppm as an initial target.) So, I hear your concern about nanoplastics, but…
https://democraticunderground.com/1127170495
At our current rate of emissions we will hit 450ppm in a little less than 15 years. That does not mean that all of the glaciers will disappear overnight. It means that without some way of lowering atmospheric CO₂ levels extremely rapidly… it’s not going to be pretty.
OnlinePoker
(5,832 posts)Carbon is a natural building block in nature, so all you would have is a literal carbon sink even if it was buried in the ground as waste and allowed to break down over time.
OKIsItJustMe
(20,735 posts)…
Nanomaterials 2021, 11(3), 745; https://doi.org/10.3390/nano11030745
Submission received: 11 February 2021 / Revised: 11 March 2021 / Accepted: 13 March 2021 / Published: 16 March 2021
(This article belongs to the Special Issue Engineered Nanomaterials Exposure and Risk Assessment: Occupational Health and Safety)
Abstract
Carbon nanotubes (CNTs) and carbon nanofibers (CNFs) are erroneously considered as singular material entities. Instead, they should be regarded as a heterogeneous class of materials bearing different properties eliciting particular biological outcomes both in vitro and in vivo. Given the pace at which the industrial production of CNTs/CNFs is increasing, it is becoming of utmost importance to acquire comprehensive knowledge regarding their biological activity and their hazardous effects in humans. Animal studies carried out by inhalation showed that some CNTs/CNFs species can cause deleterious effects such as inflammation and lung tissue remodeling. Their physico-chemical properties, biological behavior and biopersistence make them similar to asbestos fibers. Human studies suggest some mild effects in workers handling CNTs/CNFs. However, owing to their cross-sectional design, researchers have been as yet unable to firmly demonstrate a causal relationship between such an exposure and the observed effects. Estimation of acceptable exposure levels should warrant a proper risk management. The aim of this review is to challenge the conception of CNTs/CNFs as a single, unified material entity and prompt the establishment of standardized hazard and exposure assessment methodologies able to properly feed risk assessment and management frameworks.
…
orthoclad
(4,728 posts)A quick search turned up a lot of material on carbon nano toxicity, such as this from the American Chemical Society:
"The underlying mechanisms of CNT toxicity include oxidative stress, inflammatory responses, malignant transformation, DNA damage and mutation (errors in chromosome number as well as disruption of the mitotic spindle), the formation of granulomas, and interstitial fibrosis. "
https://pubs.acs.org/doi/10.1021/ar300028m
Carbon is highly reactive.
I'd say they would have to be careful of generating toxic compounds, especially if it goes into building materials and other public-facing uses.
That said, this is promising. We could replace many uses of plastic and metal with carbon, reducing the large energy budget for refining metal plus the horrific mess of plastic pollution. We'd have to be careful, though.
orthoclad
(4,728 posts)Catalyzed reactions take less energy than uncatalyzed.
We'd have to do an energy budget to compare the energy cost of the two catalyzed reactions in this process with the energy released by burning carbon sources - or with the energy captured by having that carbon in the atmosphere. The article says that use of catalysts reduces the temperature needed for the reaction from 1000C to 400C. That's still a lot of heat.
“It’s very unrealistic for large-scale CO2 mitigation [referring to the 1000C process],” Chen said. “In contrast, we found a process that can occur at about 400 degrees Celsius, which is a much more practical, industrially achievable temperature.”
OKIsItJustMe
(20,735 posts)Regardless, I don’t think we will be producing enough to have a significant effect on atmospheric CO₂.
orthoclad
(4,728 posts)"Catalysts enable pathways that differ from the uncatalyzed reactions. These pathways have lower activation energy. Consequently, more molecular collisions have the energy needed to reach the transition state." (bold mine)
https://en.wikipedia.org/wiki/Catalysis