Environment & Energy
Related: About this forumAtmospheric Mercury Derived from Coal Combustion and Cement Manufacture in China (2015).
I'll post an interesting excerpt from this paper, without too much comment: Atmospheric Mercury Concentrations and Isotopic Compositions Impacted by Typical Anthropogenic Mercury Emissions Sources Chuan Wang, Shaochen Yang, Ruolan Li, Junyao Yan, Yanxin Hu, Chuyan Lai, Zhonggen Li, Ping Li, Leiming Zhang, and Xinbin Feng Environmental Science & Technology 2024 58 (38), 16855-16866.
The introductory paragraphs are what I'll post:
Coal-fired power plants (CFPPs) and cement plants (CPs) are the predominant sectors of anthropogenic Hg emissions in China. (3,9,10) In 2015, cement production emerged as the largest source of Hg emission in China, emitting 144 Mg Hg, (11) while CFPPs constituted the second largest source, emitting 73 Mg Hg. (12) Up to 84.5% and approximately 25% of the total Hg emission amounts mentioned above are in the form of HgII from cement production and CFPPs, respectively. (11) This implies that Hg deposition from CPs is much higher than that from CFPPs in China due to higher cement-related HgII emissions. Considering that the sum of atmospheric Hg emissions from CFPPs and CPs accounted for approximately 42% of the national total emissions, (3,10) it is essential to gain a complete understating of the speciated Hg emissions from these two source sectors to comprehensively assess the subsequent environmental impacts.
"Mg" here is Megagram, i.e. tons.
Mercury, of course, has profound neurological toxicity because of its substitution, if I recall correctly, in certain zinc centered metalloenzymes important in mitochondria, a trait it shares with its congener cadmium which is also neurotoxic. Nerve cells require a great deal of energy.
Ynalvez, R., Gutierrez, J. & Gonzalez-Cantu, H. Mini-review: toxicity of mercury as a consequence of enzyme alteration. Biometals 29, 781–788 (2016)
One side effect of mercury toxicity in humans is insanity.
Mercury does not have a half-life. One isotope, 198Hg when exposed to gamma rays on an energy found in nuclear fission, will emit a neutron and decay via electron capture into elemental gold's only stable isotope, 197Au, which to my knowledge is the only way to destroy mercury without it decaying into another toxic element such as thallium or lead.
Since mercury doesn't have a half-life except under extreme conditions, in the core of a nuclear reactor (where it is rarely if ever found) mercury release is permanent.
Again, it makes people insane. I think it was insane for a certain prominent nation in central Europe, the one between Poland and France to replace its nuclear plants with coal plants, but that's just me.
I trust you're having a nice evening.
eppur_se_muova
(37,224 posts)... which was used to make hats.
https://archive.cdc.gov/www_cdc_gov/niosh/updates/upd-03-04-10.html
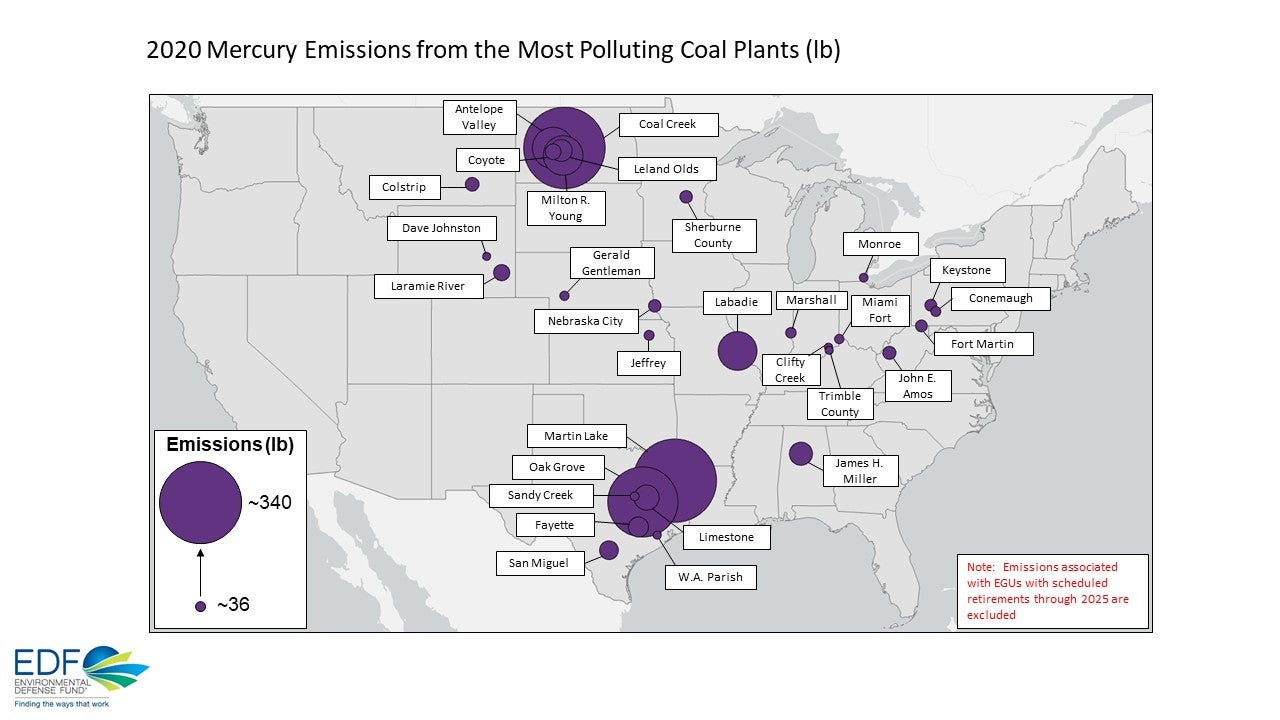
Industrial use of mercury has been phased out almost as much as possible, leaving coal combustion as the principal source of mercury pollution in the US. Not bad enough that coal causes acid rain (from sulfur and nitrogen in coal) and CO2, the principal GHG, it's also the biggest source of radioactive pollutants - worse than nuclear waste, or reactor emissions. And unlike nuclear reactors, it's not even a necessary part of the plant's operation - just unavoidable.
NNadir
(34,344 posts)I always thought it was from the practice of using mercury to brighten hat pins, with the mercury sometimes being spread over the surface by use of the hatter's lips. I'm not sure if that's true though; your explanation seems more likely.
Metallic mercury needs to be oxidized to exhibit toxicity as I understand it. Thus the toxicity of metallic mercury is likely to be accumulated over the long term, not immediately, as the mercury is oxidized to the Hg(II) oxidation state.
Mercurous chloride, an Hg(I) salt, Hg2Cl2 is only very sparingly soluble, of course, and was once used as a therapeutic agent for gastrointestinal disorders, called calomel. I believe my parents may have had some. I do know as a child, when thermometers broke, my parents, neither of whom graduated from high school - my father barely graduated from junior high school - allowed me to play with the mercury on the floor before they swept it up. Whether this had neurological effects on me is unclear to me; I am who I am.
It has been proposed - I'd do things differently since I don't consider fission products to be waste - to sequester radioactive 129I as the Hg(I) salt, Hg2(127+129)I2 salt, one of the most insoluble salts known. In most commercial regimes now or previously utilized to reprocess used nuclear fuel, it's simply been released into the environment, because of it's extremely low radiotoxicity did not justify the cost of recovering it. Over tens of millions of years, the decay of 129I would slowly release metallic mercury, preventing oxidation over all of the element to the soluble Hg(II), since Hg(II) is reduced to Hg(I) by the metal.
I wrote a sardonic essay about this over at DKos where I was ultimately banned for telling the truth, that opposing nuclear power kills people:
Radioactive Isotopes from French Commercial Nuclear Fuel Found In Mississippi River.
An excerpt:
Bullshit. I contend that if the number of people who have died from French radioiodine is not zero, it is very, very, very, very close to zero. Suppose that to prevent the release of radioiodine we required those nasty French to spend 100 million dollars to capture and contain all of their iodine. How many lives would be saved? One, maybe two, if that. Now ask yourself how many lives could be saved by donating 100 million dollars to an AIDs prevention program in Zimbabwe. I am morally averse to putting a 100 million dollar price tag on one life just because that life might be injured by a nuclear related event.
Personally, I'm rather fond of 129I and can think of better uses for it than dumping it, in particular as a source of valuable xenon. That isotope, 129I, has some interesting nuclear properties.
eppur_se_muova
(37,224 posts)I used the latest from Wikipedia. There have been times when an article at Wikipedia has changed fairly drastically between visits, so confidence level is "modest". The etymology of "Diet"* (Dutch word for Parliament/Congress equivalent) has changed before, as has that of "hepcat".
For real horror, try looking up "ormolu". I've seen some expensive antique mantle clocks at auction that were gilded in this way.
*Pronounced like DEET, the insect repellent. Also used in Japan, where it was borrowed it from the Dutch East Indies back in the day. (Originally, I was looking for info on the "Diet of Worms", where those words are NOT in English.)