Environment & Energy
Related: About this forumMy Son's Other Christmas Present For Me: Rocks to Save the World.
I remarked over in the history forum that my family gave me some gifts offering a shred of political hope: To Assuage My Despair For America: Two History Books My Family Gave Me for Christmas.
My youngest son, the nuclear engineering Ph.D. student, gave me Fears of a Setting Sun
He also gave me this: Uranium Ore Fragments

I have argued, and will continue to argue, that it may not be necessary to mine uranium for centuries if we recover the energy in so called "depleted uranium" using breeder systems and lanthanide (rare earth) mine tailings containing significant quantities of thorium.
This said, I rather like the gift, since uranium is the key to saving the world, even if his Maggotcy King Musk is a threat to losing the world.
There's nothing wrong with having a shred of hope.
Silent Type
(12,412 posts)eppur_se_muova
(41,308 posts)I have some U and Th in my garage, whose floor slopes outward, so Rn doesn't accumulate.
Open one of those jars next to a Geiger counter in several months, and you may find you can 'pour' the Rn over the detector and drive the count way up. (Remember, U ore has been in place for a LONG time and may have accumulated lots of daughter elements, including immediate precursors to Rn.)
I've read that concentrated samples of U build up a slight internal pressure over time, but that's due mostly to He, not Rn.
This all started with a serendipitous observation by the late, great, husband-and-wife team, the Jaworowskis. He, a physicist, was trying to find the source of errors in a radiation detector by calibrating it to zero at home, away from the sources of radiation in his lab. She, a paleontologist, had some fossils on her desk which she was examining before preparing a published description. He found the background radiation levels at home higher than in his lab, and traced the source to her fossils. https://www.nature.com/articles/214161a0
I've read that some fossil hunters carry radiation detectors with them, since large bones may be associated with pockets of unusually high radiation. In at least one case, it worked (ceratopsian skull -- one of the most massive bones of any animal, ever).
NNadir
(37,535 posts)...radon in my basement. I used to monitor it when I first moved in; but stopped doing so about 20 years ago. A few times I was near, but still below, the "action limit." I live near the Pennsylvania border, near the Reading Prong, a large uranium deposit that is now being fracked, accounting for the radium in flowback water, which is actually more radioactive than the sea outside Fukushima.
When they're done fracking, maybe the uranium can be removed and recovered in an ISR (in situ recovery) approach. I've fantasized about doing this with a supercritical carbon dioxide solvent.
In any case, radon or not, I'm still alive, an old man now.
This exercise with the ore led me to do something I've always wanted to do, but never found the time to do; calculate the specific activity of 238U in secular equilibrium with its decay products, leaving out a few of the insignificant branch ratios.
As I calculate it, the activity of the uranium content, (not the ore, which has other constituents) comes to about 9.27 X 106 Bq per gram, or 2.5 microcuries. It's nothing to write home about, particularly because it's alpha radiation, with a few gammas thrown in for fun.
The specific activity of a 70 kg human being from 40K, with potassium being of course, an essential element, is less, about 4.24 X 103 Bq, but it is high energy and internal (and essential) to flesh. I haven't eaten the ore, but people do it, whether they know it or not, to a very limited extent. Many phosphate rocks mined for fertilizer are low level uranium ores. In fact, there was thought, in the 1950's of mining these rocks for uranium, until richer ores were discovered. These rocks apparently come from New Mexico.
eppur_se_muova
(41,308 posts)The bottle leaked, the plastic bag didn't. Don't know how long that would have held up, and it had to be disposed of anyway. ![]()
Bummer about living near such a radon source.
"actually more radioactive than the sea outside Fukushima" ... Well, I think we both know how low a bar that is. ![]() Too bad journos never get it.
Too bad journos never get it.
I remember (well, at least generally) when you posted about NORM in used drilling mud. I hadn't realized that was something that affected you personally, just realized that was one of those things that most people never worry about. Unintended Consequences and all that.
I wonder if the air in a basement could be continuously pumped through refrigerated water, and the radon hydrate allowed to settle to the bottom. I once saw a demonstration by an MIT chemist of the effect of bubbling Xe through H2O (maybe D2O, not too important) and MRI'ing the result. There was a layer of Xe-rich water towards the bottom, and pretty much Xe-free above that. I keep thinking that someday this will be used to collect Xe isotopes from nuclear reactors, but if anyone's doing that, I haven't heard of it. Of course you'd have to remove everything reactive first, so maybe that's unworkable. There are lots of pubs on Xe-129 and Xe-131 NMR of hydrates; maybe I need to d/l and read a few. It's something that's been on the back burner for years. If you've heard more on that, let me know.
NNadir
(37,535 posts)He's in town for the holidays. The topic involved a certain kind of passive reactivity control using fissiogenic molten CsI which has a fairly high neutron capture cross section for the two I and two of the Cs isotopes, but can be, in effect, a burnable poison. The product of neutron absorption in 127I and 129I would be 130Xe, and 128Xe. 129Xe, as you note, is magnetically active in NMR, and it is a shame that the half-life of 129I is so long, since it would be a source of this nucleon. It has been used in a medical setting in MRI for lung diseases, but I think it's very expensive.
As for burnable poisons, my son tolerates my ideas. He may appreciate them more when I'm dead. (I hope so.)
There has been a number of discussions of recovering xenon from used nuclear fuel; the key being separating it from fission product krypton which includes radioactive 85Kr (t1/2 = 10.76 y).
In fact, some time ago, I attended a lecture on the topic at Princeton University, which was surprising, because in general, Princeton University is an intellectual anti-nuke hellhole except for dreams of fusion, fusion obviously having been far too late to do a damned thing about extreme global heating now being observed. At Princeton University, they want to wait, like Godot, for the so called "renewable energy" nirvana that is not here, did not come, and won't come.
The lecture was by this guy: Moises A. Carreon
I was surprised as hell that there was actually a discussion at Princeton on used nuclear fuel reprocessing. Maybe it was an attempt to be "fair and balanced;" I don't know.
A paper reflecting the topic he discussed is this one:
Microporous Crystalline Membranes for Kr/Xe Separation: Comparison Between AlPO-18, SAPO-34, and ZIF-8 Ting Wu, Jolie Lucero, Zhaowang Zong, Sameh K. Elsaidi, Praveen K. Thallapally, and Moises A. Carreon ACS Applied Nano Materials 2018 1 (1), 463-470.
85Kr could be made to make a photoelectric battery in theory, since ionized Kr (like Xe) has the property of producing a glow very much like daylight, and in fact, is used as such in some lighting applications; I believe some expensive headlights on cars use this approach. There will never be enough 85Kr to make this a major energy source, because of secular equilibrium, but one can imagine specialized areas in which it might have application. Interestingly, the decay product would be the only source of nonradioactive rubidium, 85Rb, since natural rubidium, like natural potassium, is radioactive owing to the very long lived 87Rb isotope that is used in radioactive dating of very old rocks.
It appears, that in the ocean, where 222Rn is formed from the 4.5 billion tons of uranium contained therein, a disequilibrium exists because of the low solubility of thorium in seawater, as well as outgassing of 222Rn, apparently unrestrained by clathrate formation.
This topic is covered in a wonderful book I obtained through the interlibrary loan program at my public library, and scanned in a searchable PDF at Princeton, antinuke hellhole, in defiance of their stated policies of waiting for Godot.
U-Th Series Nuclides in Aquatic Systems
Apparently clathrates may not apply quite as well to radon as to xenon, since the ocean outgases radon as does groundwater.
From the text:
Groundwater can be another source of'222Rn to the atmosphere. Groundwaters typically have 222Rn activities in the range of a few hundreds to a few thousands of dpm/L (Porcelli, this volume). Use of groundwater for agricultural, domestic and industrial purposes would release 222Rn from them to the atmosphere. The flux of Rn from this source could have significant spatial variability and its importance relative to diffusion from soils would depend on the magnitude of groundwater usage. Hussain and Krishnaswami (1980) estimated that in Ahmedabad, a major urban area of India, the flux of 222Rn associated with groundwater usage is a few percent of the soil-derived flux.
cf. ibid, Chapter 2, Church and Sarin, U and Th Series Nuclides in the Atmosphere: Supply Exchange, Scavenging and Applications to Aquatic Processes, Page 14
Figure 2:
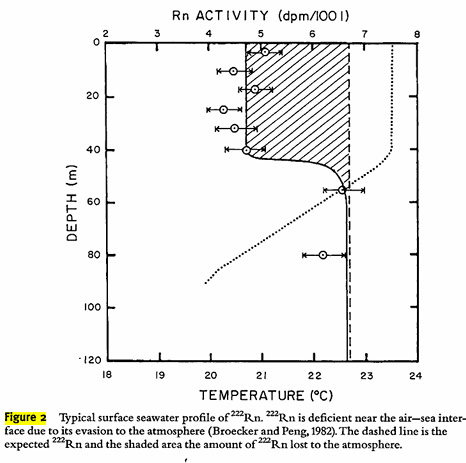
This may be a function of temperature. I have not looked into Rn clathrates to be honest.
Thanks for your comment.
