Science
Related: About this forumA systematic "omics" (molecular biology) approach to the effects of radiation on living tissue.
The paper I'll discuss in this post is this one: Multiomics Point of Departure (moPOD) Modeling Supports an Adverse Outcome Pathway Network for Ionizing Radiation You Song, Keke Zheng, Dag Anders Brede, Tânia Gomes, Li Xie, Yetneberk Kassaye, Brit Salbu, and Knut Erik Tollefsen. Environmental Science & Technology 2023 57 (8), 3198-3205.
All of our regulations concerning the effects and risks of exposure to ionizing radiation are based on work conducted on rats in the late 1940's at Oak Ridge National Laboratory, well before the science of molecular biology really existed at anything close to the level we see today, at least as a science with a modicum, of depth.
It was, for example, before working with the diffraction results obtained by Rosalind Franklin, Watson and Crick developed and published the famous double helix structure of DNA.
Today the science of molecular biology, developed using modern tools like high resolution mass spectrometry, high field multidimensional NMR (COSY, NOESY, HSQC), PCR, qPCR and advanced computational capabilities is a highly developed science. The number of these techniques that existed in the 1940's is zero. (X-Ray diffraction was known, but not applied in any capacity in the rat study of the effect of radiation on rats.)
The 1940's "science" on which our radiation regulations are based is known as the "Linear No Threshold" (LNT) hypothesis; which has killed people in vast numbers led by ignoramuses like, say, Ralph Nader, to make very stupid claims that "there is no safe level of radiation." Nuclear energy saves lives, and it follows that anti-nuke ignorance kills people.
Despite the almost religious dogma attached to the LNT hypothesis, actually, there is a level of radiation below which everyone on the planet would die. All of the potassium on Earth is slightly radioactive, owing to the the presence of the long lived isotope K-40, which has a half-life of 1.227 billion years. Potassium is an essential element. Hypokalaemia, the medical term for potassium deficiency, sometimes observed from dietary restrictions or deprivation. and/or the use of certain medications, like diuretics, has serious health consequences, in extremis, death. As a result, a healthy human being weighing about 70 kg will necessarily contain, internally, about 3500 Bq of radioactivity.
Modern health physicists have seriously questioned the LNT, with good reason, led by Dr. Ed Calabrese, who was recently interviewed in a thirteen episode video series, available on the internet from the Health Physics Society that can be found here: The History of the Linear No-Threshold (LNT) Model Episode Guide
When my son, a materials scientist, began to embark on his Ph.D. program in nuclear engineering, I advised him to watch this series, which he did, which may have contributed to his high grade in his required graduate course in radiation safety.
The stupid rhetoric proposed by scientifically illiterate attorneys like Ralph Nader, the claim that "there is no safe level of radioactivity" is very popular to this day. Recently over in E&E, there was a very stupid post about how the release of extremely low level tritiated water from the regrettably closed once life saving Indian Point nuclear reactors into the Hudson River was some kind of tragedy. The moronic rhetoric therein included a statement of the population of New York City, as if the city was going to be wiped out by this event.
Americans like to compete to see who can make the most spectacularly paranoid and hysterical misinterpretations of environmental events and events and technologies health consequences, ranging from the "safety" of mRNA vaccines (a scientific triumph of the first order) to, most recently, the question of whether the recent train crash in East Palestine Ohio will leave that part of Ohio permanently uninhabitable. (The most stupid post here on that subject at DU focused on the putative formation 2,3,7,8-Tetrachlorodibenzodioxin, aka "agent orange" and a conspiracy theory involving the EPA, the agency that developed the standard analytical methods for its detection.)
As for the putative effects of the release of low level tritiated water into the Hudson River, it anything ever wipes out New York City or at least makes it uninhabitable, it will be climate change. The assholes who carry on about things like this, who post stupid pictures of hydrogen cars and appalling and disgusting pictures vast stretches of industrialized former wilderness covered by semiconductors to power energy wasting electrolysis units, don't give a rat's ass about climate change, never have, never will. Nor do they give a rat's ass about all the people who died yesterday, will die today and will die tomorrow from air pollution in New York City, in New Jersey (where I live) or anywhere else on the planet. The number of citizens of the City of New York who will die from tritiated water released into the Hudson River at Indian Point will be zero. By preventing the combustion of dangerous fossil fuels and the resulting air pollution, Indian Point saved the lives of New Yorkers.
Happily, with respect to LNT assumption and the associated mysticism, things are changing. As opposed to the "science" that generated the LNT - Dr. Calabrese argues vociferously that the Oak Ridge rat studies were sloppy and ridden with objectionable bias, even fraud - modern molecular biology tools are finally being applied to examine the assumptions underlying cult like worship of the LNT.
"Omics" refers to the subdisciplines of molecular biology, generally broken into classifications defining the subclasses of biological molecules: Proteomics refers to the biology, chemistry and physics of proteins; glycomics refers to biology, chemistry and physics of sugars, lipidomics refers to biology, chemistry and physics of lipids (aka "fats" ); and genomics refers to the biology, chemistry and physics of nucleic acids, particularly the forms and functions of RNA, as well as DNA. It must be clear that these subdisciplines overlap profoundly. One cannot really understand proteomics for instance, without paying attention to glycomics, since many proteins derive their functional attributes from being glycosylated, having often very complex sugars attached to them.
There is a fantasy that all of these molecules must be free of insult, that their structural integrity - this is particularly a widely held popular belief associated with DNA - must be maintained invariably or serious health effects will inevitably result. In many cases, there is nothing "inevitable" about structural variations or damage to DNA; organisms have evolved very sophisticated systems of DNA repair. In cases where DNA damage has resulted in cellular dysfunction, multicellular eucaryotic organisms - this class includes human beings - have mechanisms for inducing apoptosis, the death of those cells. One should expect that everyone regularly generates cancer cells; happily in most cases these cells are addressed and killed by an immune response. (Modern cancer therapies include addressing the immune responses to cancer cells that evade this immune response, for example the class of drugs known as "checkpoint inhibitors" turn off the disguises that cancer cells use to evade immune responses.)
The nucleic acids, including the proteins they encode, are dynamic molecules. They cannot function without constant and regular and (mostly) regulated change.
Changes to DNA are inevitable. One of the drivers of aging is believed to be the methylation of DNA; in this case the changes to DNA do have inevitable health consequences: Death.
I will die. You will die. We all will die. What matters is not that we will die; but that we live, and beyond merely living, how we live, our access to those things that fill us with wonder, excitement, love, health and well being, and, finally, although this seems less and less important to our societies as a whole, the world we leave after we are done with it.
There are, of course, people who do not live well, cannot live well or even decently. It's called "poverty." It matters, to me anyway, if not to everyone.
The main point is this: Changes to biological molecules, including those caused by environmental insults can and do lead to health consequences, but often they do not, because life, as a necessity for its continuity, has evolved very sophisticated mechanisms to restore homeostasis when it is disrupted. Arguably birth is a form of establishing the homeostasis of the biome. Indeed, it is a canon of medicinal chemistry, more and more directed by molecular biology than by empirical discovery, that diseases can be cured by stimulating these mechanisms, as in the example of the checkpoint inhibitors discussed above, and, in fact, the mRNA vaccines, or in other cases by mimetics of the working of these mechanisms, for example, antibody drugs.
Before discussing the paper mentioned at the outset of this insufferable post, let me discuss, besides radiation, an alternate means of insult to biomolecules, in this case DNA, to which I have a personal connection, since it was involved in the death of my uncle and my father in ways that I did not understand at the time of their deaths but that I understand now. I seem to have inherited the happily unusual feature of their genome that was involved in killing them and have been sure to advise my own sons of this, this to tell them to avoid certain behaviors and their exposures.
While the following paper, which I came across when preparing for a talk I was giving, is not specific to the particular case of my father, my uncle and myself, it does touch on the general mechanism by which the responses to their genome graduated to cancer, and in my case has not (yet), done so.
The paper is this one: Products of the Direct Reaction of the Diazonium Ion of a Metabolite of the Carcinogen N-Nitrosomorpholine with Purines of Nucleosides and DNA Charles N. Zink, Nicolas Soissons, and James C. Fishbein Chemical Research in Toxicology 2010 23 (7), 1223-1233.
Here is the opening excerpt of the introduction:
Most people, particularly those who go into that Atkins diet stuff, understand that protein rich foods are meats; nitrate rich foods are semi-preserved meats, bacon being the classic case, although things like beef jerky for another example, qualify, as probably do salami, certain kinds of preserved ham and probably other foods. I haven't eaten any of these things for over 40 years, but I certainly grew up eating them. They were staples at my childhood breakfast table.
Scheme 1, which is just a bunch of chemical structures that make sense to me but may not make much sense to people who have not been exposed to college level chemistry classes:
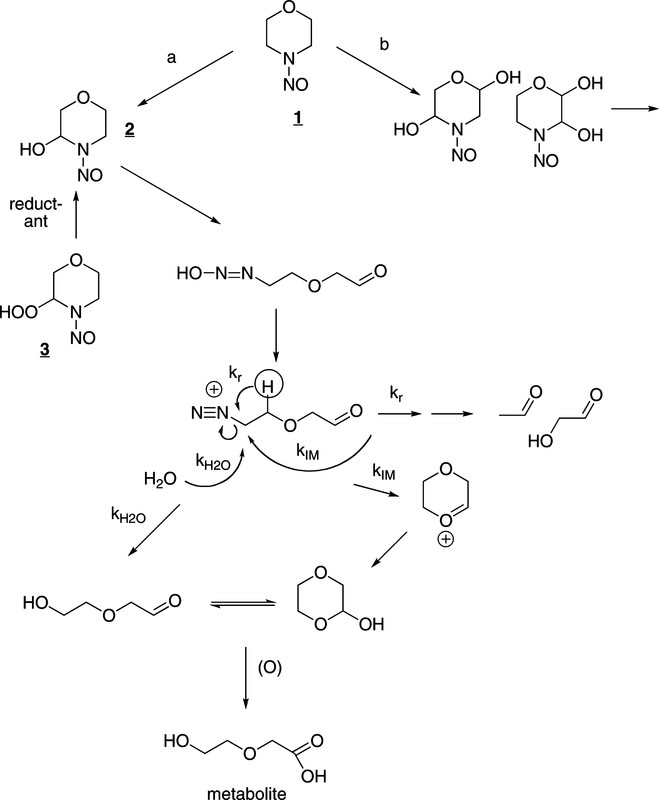
The operative molecule is the one in the center, shown in the act of decomposing by using the arrows. It is metastable; it exists only for a brief time being very reactive, but it the reactivity that is problematic.
The paper continues with some chemical jargon:
There is good evidence from studies of the metabolism of the radiolabeled compound that a significant part of the metabolism involves simple α-hydroxylation, path a in Scheme 1, as indicated by the isolation of the metabolite, bottom of Scheme 1 (18, 19). Largely through the efforts of the Loeppky group, a second pathway involving β hydroxylation has been elaborated (path b, Scheme 1) (20-26). This pathway gives rise to an overlapping set of DNA damaging agents some of which have been characterized. While it was reported earlier that α-hydroxyNMOR, 2, yields “almost entirely” acetaldehyde (27, 28), this was later demonstrated to be incorrect (29). About one-quarter of the diazonium ion derived from α-hydroxyNMOR does undergo rearrangement (kr, Scheme 1) generating acetaldehyde and glycoaldehyde. Predominantly though, α-hydroxyNMOR forms 2-hydroxyethoxyacetaldehyde or its cyclic form by either the kH2O and kIM pathways, respectively, in Scheme 1.
Anticipation that direct reaction of the diazonium ion in Scheme 1 might give rise to DNA adducts, classical nitrosamine damage, is tempered by the extensive studies of Hecht on the DNA adducts from N-nitrosopyrrolidine (NPYR) and N-nitrosopiperidine (NPIP) (30-40)...
The mechanism of alkylation of purine bases in DNA is shown in the text included in this graphics object from the paper:
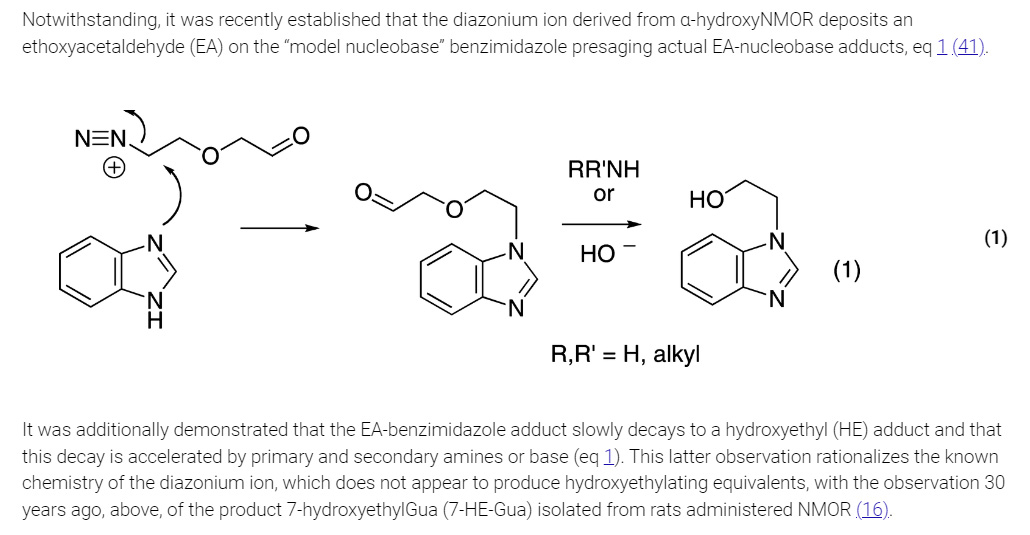
The authors of this paper synthesized a series of analogues of the nucleobases guanine and adenine found in DNA for analytical standards for LC/MS/MS:
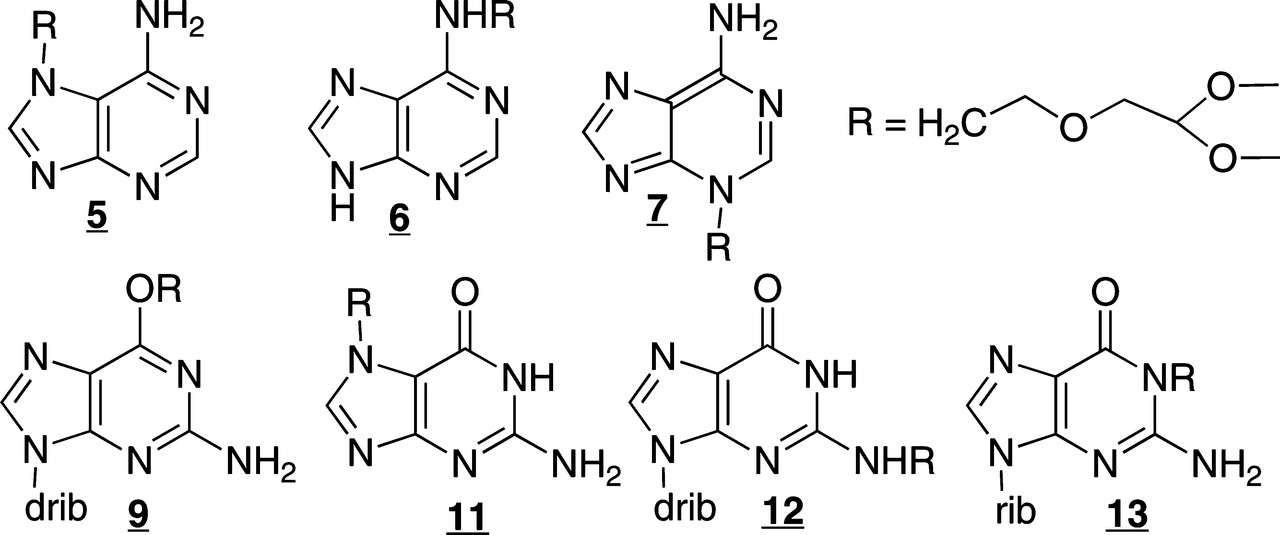
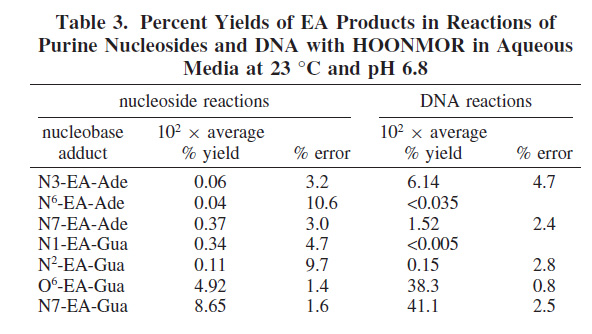
They then exposed DNA, in vitro (cell free) to the NMOR carcinogen to demonstrate the type of modifications were found via alkylation.
The results are shown in this table:
When my father got his first cancer, he told me that he could believe smoking was the cause of his cancer - both he and my uncle were heavy chain smokers - if he got lung cancer, but since he didn't have lung cancer the cause was something else. (My father had an 8th grade education.) Having been informed of his diagnosis before flying home to see him, I had already hit the books and I politely informed him, somewhat smugly, that there was three known causes of the cancer he had, one being a heavy drinker, the second being a heavy smoker, and the third being a citizen of a region in Pakistan, and then asked him which one applied to him. (This was almost two decades before the Chem Res Tox paper was published and the time I personally knew very little about the alkylatiion of purine nucleobases at the time; I was a smart assed kid.)
His cancer - caused by smoking - killed him, showing up in his lungs as well before the end.
There is a specific tobacco related nitrosamine that works like the NMOR above; it is known as NNK and alkylation of nucleobases in vivo - in rats - has been demonstrated in very modern nanoLC nanoelectrospray HRMS. (cf. Analysis and Identification of 2′-Deoxyadenosine-Derived Adducts in Lung and Liver DNA of F-344 Rats Treated with the Tobacco-Specific Carcinogen 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone and Enantiomers of its Metabolite 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol,
Erik S. Carlson, Pramod Upadhyaya, Peter W. Villalta, Bin Ma, and Stephen S. Hecht Chemical Research in Toxicology 2018 31 (5), 358-370). This particular nitrosamine is so reactive that it alkylates all four of the DNA nucleobases and, even more dramatically, the phosphate linkers connecting the deoxyribose.
When I saw my gastroenterologist for the first time on an unrelated issue he asked about my family history and when I explained how my father died, he asked if anyone else in my extended family had also developed this cancer and I indicated my uncle had as well. He then suggested that I be scoped for the same cancer. Being a male, and prone to denial, I indicated that both my uncle and my father had been heavy smokers and that I didn't smoke and therefore a scan was unnecessary. He insisted that he knew more about the subject than I did - of course he was right - and that I should not ignore the possibility of a genetic condition. He prevailed; I had the scope, and it turned out after a biopsy that I had the precancerous condition that would lead to the same cancer that killed my father and uncle. At the doctor's recommendation, I went back six months later for a recheck, whereupon the same tissues biopsied the first time had reverted to normal. In other words, my precancerous lesions healed, spontaneously, without treatment. That happens. It's not a miracle. It's biology.
There is a point to this personal tale and it is this: It is quite possible that I have generated cancer cells multiple times as a result of exposure to ubiquitous nitrosamines other than NNK. (It's possible I've been exposed to NNK from second hand smoke; I certainly was in my childhood. My living room smelled like an ashtray when I was growing up.)
My father smoked from the time he was eight years old until his death in his early sixties. (He was still sneaking smokes after his cancer surgeries.) His older brother had a similar smoking history. Both lived more than 50 years as heavy smokers, breathing in and swallowing a highly carcinogenic compound, NNK. Both lived long enough to survive wars, get married, raise children in houses they bought. Over this expanse of time, it seems unlikely that cancer cells that ultimately killed my father and his brother were the first cancer cells that either of them experienced. The ones that killed them were merely the first that were able to evade the repair mechanisms that most living things, and even some nonliving viruses, contain; in the human case this repair mechanism is a very complex and elaborate, highly evolved, immune system. Note that it need not be the genome of the cancer cells themselves that allows for the metastasis and survival of cancer cells. It is quite possible that the cells damaged are those that generate responses to cancer cells.
As is the case with vaccines, the immune system works best when stimulated. I'm not here to advertise for the largely discredited theory of homeopathy, but in a sense, the "hair on the dog that bit you" is not entirely a bad thing, as long as its just the hair and not the dog itself. The "hair on the dog" of a vaccine is a protein sequence from an inactivated (really dead) virus, or in the case of the super modern mRNA vaccines, causing your cells to synthesize the "hair on the dog," not the dog, just the hair.
Not all cancers are caused by nitrosamines, of course, there are many other mechanisms. In the case of air pollution, the adducts are not alkylated nucleobases, but rather aromatic adducts formed by the formation of Schiff bases from quinoidal species and/or cycloadditions. Chlorinated species can react by nucleophilic substitution, etc.
In the case of radiation, radical species, in particular, those calling oxidative stress are involved in changes to the DNA. As is the case with the nitrosamine adducts (and indeed other adducts) the products of these reactions can now, with modern instrumentation, be definitely observed to glean the mechanisms by which they work.
This brings me to the paper I cited at the outset of this post.
The introductory text from that paper:
An adverse outcome pathway (AOP) has been introduced as a conceptual framework to facilitate mechanistically based risk assessment. An important characteristic of the AOP framework is that it allows for utilization of information generated by cost-efficient New Approach Methodologies (NAMs), such as in vitro high-throughput screening (HTS), high-content (HT) OMICS, and in silico modeling to support regulatory decision making. Although the AOP framework is rapidly developing for chemical safety assessment, its application to nonchemical stressors such as radiation remains to be better developed. Establishment of the Radiation and Chemical (Rad/Chem) AOP joint topical group, a sub group of OECD’s Nuclear Energy Agency (NEA) High Level Group on Low Dose Research (HLG-LDR), is envisioned to help facilitate such AOP development in radiation research and foster broader implementation of AOPs into hazard and risk assessment. (4) A number of ionizing radiation AOPs have subsequently been developed and submitted to the AOP repository AOPWiki (https://aopwiki.org/).
As a type of NAM, OMICS techniques (e.g., genomics, transcriptomics, proteomics, metabolomics, etc.) have been widely used to understand the toxic mechanisms of stressors and to develop novel biomarkers for environmental surveillance. Approaches to better utilize OMICS data to support hazard and risk assessment are also rapidly evolving in recent years. For instance, standardization of the OMICS data reporting has been proposed to meet regulatory requirements. (5−10) Quantitative approaches such as benchmark dose (BMD) modeling have been adapted to the OMICS data for estimating points of departure (POD) that are relevant for setting safety thresholds...
I have highlighted the one word which I find very refreshing. It is conceded at the outset that a threshold exists, not that there isn't any, which should be a priori obvious (but hasn't been so to regulatory agencies) since all living things are continuously bathed in ionizing radiation from a variety of sources including NORM, yet life on this planet exists, and indeed existed when the planet was far more radioactive than it is now after billions of years of radioactive decay.
A later excerpt:
...Based on the previous advances and remaining research needs, the present study was conducted with the main aims of: (1) generating new empirical data using multiomics (transcriptomics and metabolomics) analysis and functional bioassays to better understand the effects of chronic low-dose ionizing radiation, using the model aquatic crustacean Daphnia magna and γ radiation as prototypes; (2) developing a biostatistical-bioinformatic workflow for multiomics integration and POD (moPOD) estimation; and (3) investigating how a combination of multiomics and functional PODs can support WoE considerations for an AOP network (AOPN) focusing on the effects of ionizing radiation. Transcriptomics and metabolomics were combined to form a multiomics approach in this study, as (1) transcriptomics has been widely used in eco(toxicology) and radioecology to indicate early stress responses (upstream events) and understand toxic mechanisms; (2) the tPOD has been demonstrated to be useful by several studies; (3) metabolomic responses are considered more representative of phenotypic changes (downstream stress responses at the molecular and cellular level) compared to other omic responses; and (4) data generated by these two types of OMICS analysis cover both signaling and metabolic pathways that provide a relatively more holistic picture of global stress responses to radiation exposure.
The organism used in the experiments was Daphnia magna, known as a "water flea," an organism with a life span of about three months, a very small primitive crustacean that is considered a constituent of zooplankton.
The experimental details of the radiation exposure are given thus:
Mayak is the most radioactively contaminated place on Earth; it was the site where the Soviet Union isolated plutonium for its nuclear weapons, corresponding to our Hanford site. In 1957 a chemical explosion in a waste tank caused the wide spread distribution of fission products over a wide area, but this was not by any means the only cause of contamination: The Soviets routinely dumped chemical waste contaminated by highly radioactive fission products in a nearby lake that drained via a river, when not frozen, into the arctic ocean. The highest average annual personal exposure recorded at Mayak was in 1949, still in Stalinist times, not far from the demonstration of nuclear weapons capability in the Soviet Union. It 489 mGy among reactor workers equivalent to about 4 hours of the highest dose rate to which the Daphnia were exposed.
Source: Mayak Worker Dosimetry Study Project 2.4 Overview of Dose Assignment Methodology for Mayak Workers
It is important however that radiation effects are known to depend on the rate of exposure. As a year has 8766 hours, the 1949 average rate is about 0.06 mGy/hr, slightly larger than the lowest rate for the Daphnia experiment.
A note on units: mGy is milliGray. A gray is a unit of energy density, deposited energy density, a derived unit that is Joules/kg, which may also be reduced, as a joule is a kg-m^2/sec^2 to m^2/sec^2. A related unit is the Sievert, which includes a "quality factor" related to the ionizability of molecules in various tissues, generally a "whole body" average, although organ specific quality factors are well known.
This quality has bearing on the experiment itself, since Daphnia are aquatic organisms; water may be shielding, on the other hand, it is readily ionizable and generates free radicals like the highly reactive •OH radical.
At Chernobyl, in the Red Forest immediately after the accident in 1986, where trees were killed by radiation exposure, the rate of exposure was recorded as 27 mGy/day or 1.1 mGy/hr, about a hundred times lower in rate than the highest dose given to the water fleas in the experiment, but the exposure took place over many weeks and months whereas the experiment was conducted over 4 day and 8 day periods, using fairly constant exposure from Co-60 as opposed to the attenuating I-131 that dominated the early days but was supplanted by longer lived volatile isotopes, particularly those of cesium.
(S.A. Geras'kin, S.V. Fesenko, R.M. Alexakhin, Effects of non-human species irradiation after the Chernobyl NPP accident, Environment International, Volume 34, Issue 6, 2008, Pages 880-897.)
The dose rates at Chernobyl fell rapidly with the decay and diffusion of iodine-131, which has a half-life about 8 days. As of 2022, most dose rates in the exclusion zone had fallen to significantly less than 0.5 mGy/hr.
Source: Beresford NA, Wood MD, Gashchak S, Barnett CL (2022) Current ionising radiation doses in the Chernobyl Exclusion Zone do not directly impact on soil biological activity. PLoS ONE 17(2): e0263600
A flaw in the paper as I see it may be the analytical methods used, particularly with respect to the metabolomics, which utilized a somewhat less than modern GC/MS, a single quad instrument, with data generated using a commercial metabolic library, as opposed to a far more sophisticated LC/HRMS system. Even with this very modern equipment, HRMS, metabolic data can be subject to misidentification, and the topic is very much evolving via international bench marking of non-targeted substructure identification: BP4NTA
Nevertheless the authors appeal the the KEGG metabolic pathways, and thus structures that are well known.
This in mind, the excellence of the approach, applying the tools of microbiology to understand radiation, the limits of the analytical tools notwithstanding, let's look at what the authors findings were by at least looking at the pictures.
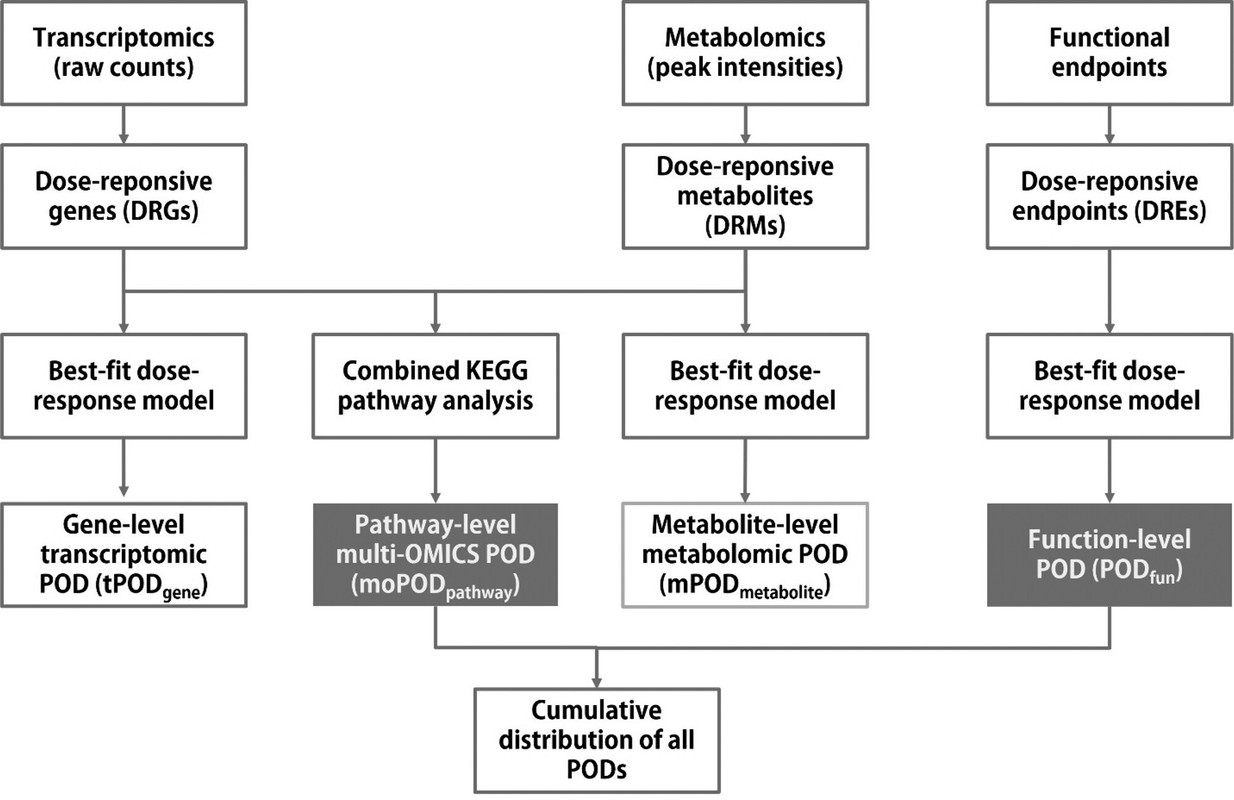
The caption:
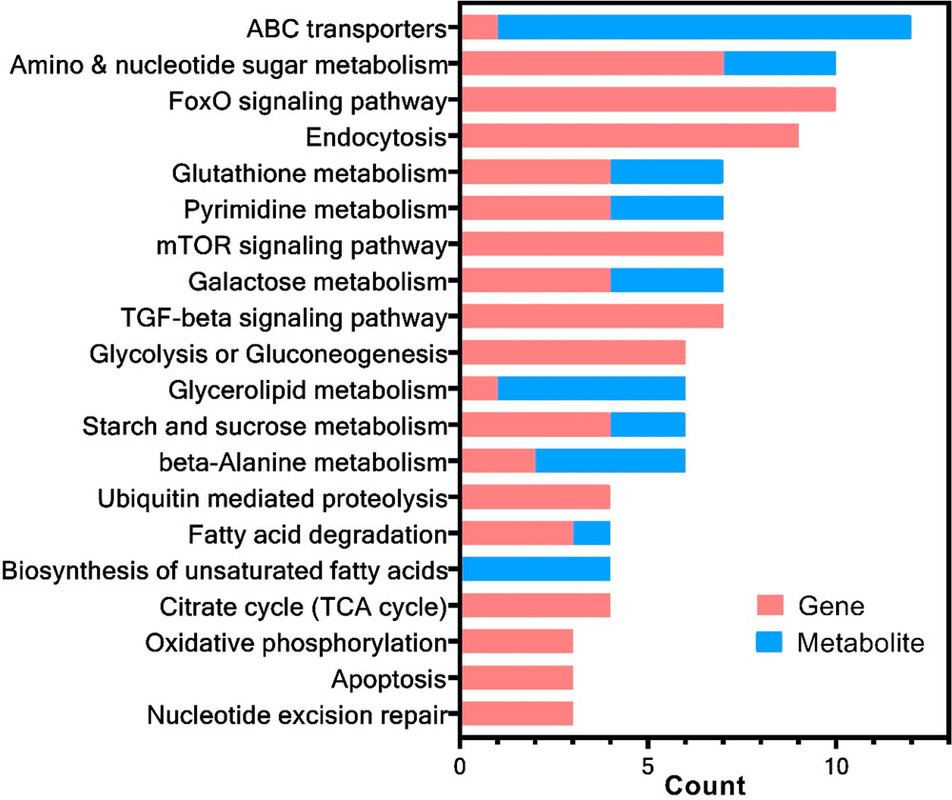
The caption:
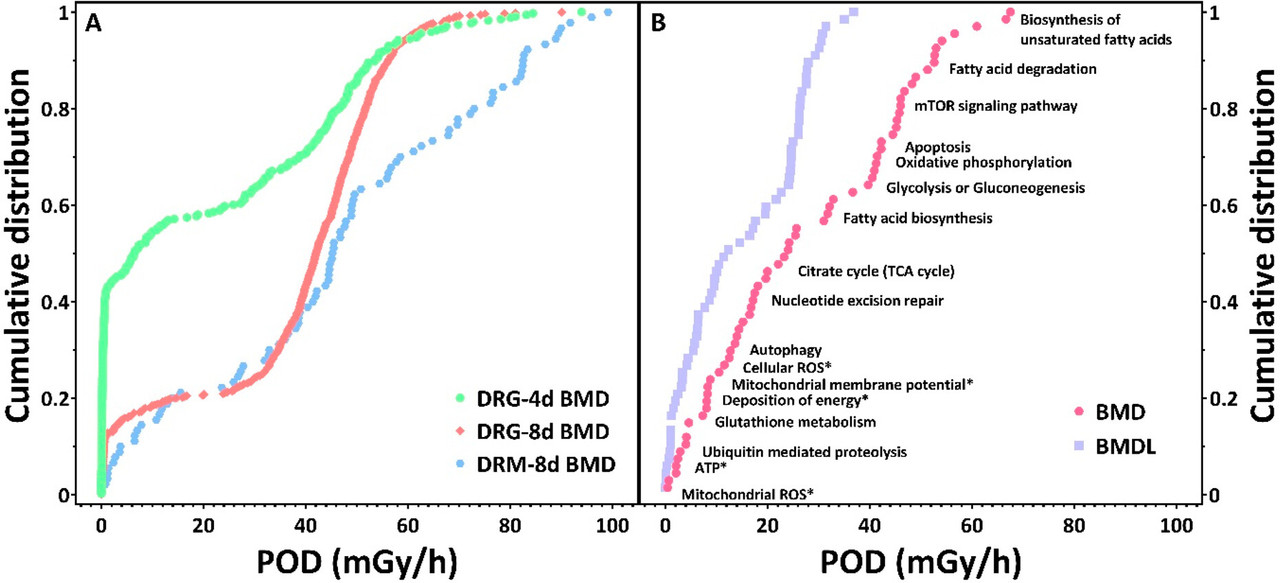
The caption:
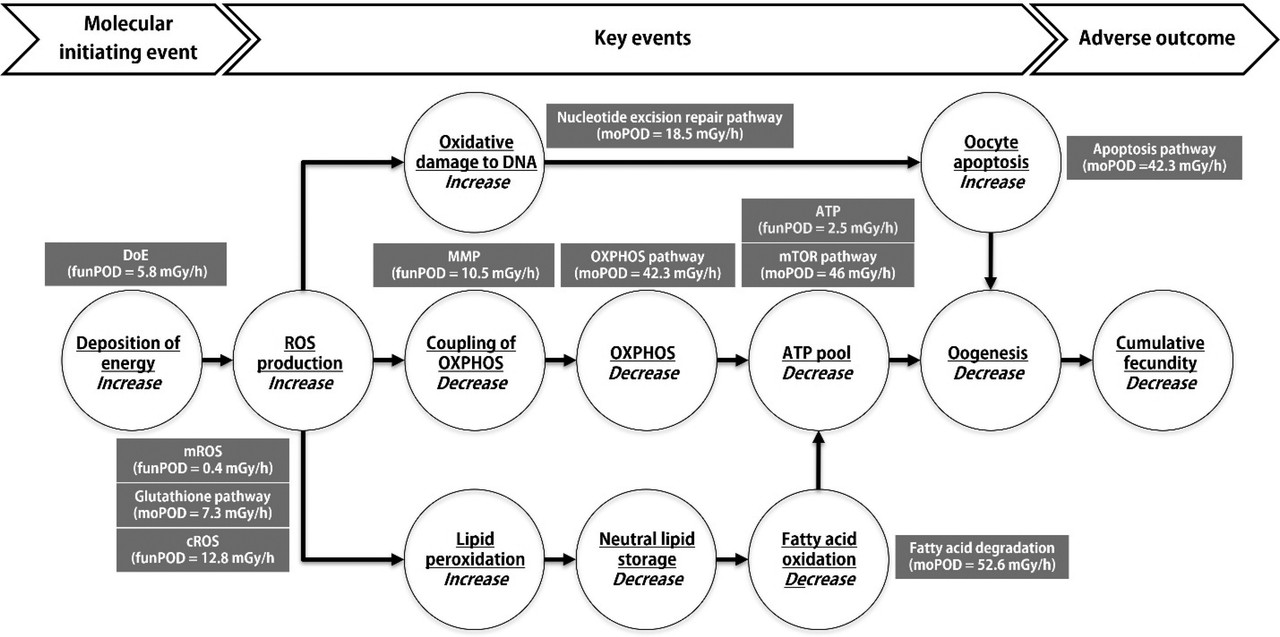
The caption:
This fun stuff aside, it is worth looking at the discussion in the text for another reason. There is a theory that exposure to low level radiation may actually in some cases be beneficial. This theory - and I'm not here to argue whether it should be accepted or not - is called hormesis. The discussion gives a possible mechanistic reason why it might be real, and I will highlight it in the author's text below:
ROS, reactive oxygen species, are a normal reality in the environment; radiation induced ROS merely represents a subset of possible origins. (Air pollution is another.) By stimulating the production of natural antioxidants found in living systems - of which glutathione is one example - "hair on the dog that bit you" can (but certainly does not always) have a protective effect; which may describe a possible mechanism hormesis to the extent that it is real, something that to my knowledge is not conclusive.
The authors note the destructive effects of radiation on a number of types of molecules and their estimation in these species is to set a BMD, a "benchmark dose" at which statistically important effects are found in 10% of the organisms. The software package for doing this, developed for chemical toxicology and not radiation toxicology, is described here:
DRomics: A Turnkey Tool to Support the Use of the Dose–Response Framework for Omics Data in Ecological Risk Assessment Floriane Larras, Elise Billoir, Vincent Baillard, Aurélie Siberchicot, Stefan Scholz, Tesfaye Wubet, Mika Tarkka, Mechthild Schmitt-Jansen, and Marie-Laure Delignette-Muller Environmental Science & Technology 2018 52 (24), 14461-14468.
The authors explain the limitations of their study:
The limitations listed include those I've listed above, the unreliability of their detection system for metabolites, and a lack of evidence describing the larger gene pool to focus on a relatively small set of genes. The part I have placed in bold reflects the fact that the organisms studied are relatively primitive and may lack, surely lack, the sophisticated repair capabilities of higher organisms. (This species, Daphnia, is notable for the degree of parasitism it experiences, suggesting at best a primitive, if any, effective immune system.)
There is, I think, a little bit too much innuendo in the paper using conditional words like "can" and "could" to describe the effects of radiation on biomolecules, but the general approach, a molecular biology approach to evaluating the risks of radiation exposure when it occurs, is something long overdue, particularly when and where better analytical tools are utilized.
It's certainly a pathway away from letting millions of people die each year from air pollution and climate change because we've never updated our regulations based on questionable experiments conducted on rats in the 1940s. To the extent that these experiments have led to the widespread adaptation and believe in rather deadly urban myths ("There is no safe level of radiation" ) they should clearly be disregarded for their sloppiness and irreproducibility alone. It's clear from data, including the life of one of the first nuclear liquidators ever, President Jimmy Carter, that the risks of radiation may not be what is advertised. One sees appeal to this pernicious mythology here all the time, when badly educated fools suggest that the disposal of a decommissioned reactor core is somehow a tragedy worthy of extreme concern, or implications that the discharge of low level tritiated water at Indian Point will lead to a huge health crisis in New York City. This kind of thinking quite literally kills people in the same way antivax ignorance kills people, by encouraging the omission of valuable procedures and processes, be they medical or industrial. The LNT has got to go if we are really interested in saving what can be saved and restoring what can be restored. Frankly the sea fleas here are far more interesting and worthy of attention than the historic rats at Oak Ridge studied about 7 decades ago.
I would hope we will build on this kind of work.
I trust you're having a pleasant Sunday.
1WorldHope
(900 posts)I have no formal, nor informal, education in the sciences. But I think someone missed the boat for me growing up. I am fascinated by physics and biology and astronomy.
That aside, I really would be interested in this being sorted out. Are you saying that nuclear energy could save us? I would be one of those fools fearing nuclear waste. If that is not true. .. then holy crap, let's talk more. How much carbon do you think nuclear energy could replace?
Fascinating stuff.
NNadir
(34,662 posts)I'm not saying "nuclear energy could save us."
I'm saying it's our last, best hope. Either we embrace it, or the world falls apart.
I have a standard answer whenever I hear about so called "nuclear waste."
I do this:
I produce a reference to the primary scientific literature, which is open sourced, detailing the death toll associated with major risks causing disease and death.
It is here:
I then produce an excerpt from the paper focusing with bold on the deaths from air pollution, which although it is never described as such is "dangerous fossil fuel waste."
I then ask anyone and everyone commenting on so called "nuclear waste" to identify, in the 70 year history of nuclear energy, to identify as many people as have died in a relatively short time period from air pollution, an hour (750 dead), a day, (around 18,000 dead), a week, (around 129,000 dead.)
I never get a response, even if the time period I use is an hour (750 dead).
If it's so damned dangerous, where are the dead?
(Used nuclear fuel is a very valuable material as it turns out, but society isn't quite "there" yet.)
You are decidedly not a fool if you question what has been drilled into your mind by rote chanting for decades, which is that "nuclear waste" or "nuclear accidents" blah, blah, blah, are somehow remotely a major concern whereas the deaths of around 70 million people a decade from dangerous fossil fuel (and biomass) combustion waste isn't, that the things about which anti-nukes chant insipidly are a concern on the level of climate change.
It's not even close. It's as absurd to say so as it is to believe any of the drivel that drools out of the mouth of Donald Trump, in short, a bald and obvious lie, but one that has entered urban mythology and has provided an enormous death toll.
One of the things drilled into people's minds, which is as nonsensical as the idea that vaccines are dangerous, is that there is no acceptable level of radiation. This is nonsense, based on what can only be characterized as very weak work performed in the 1940's, almost 80 years ago, the point of my post.
Nuclear energy is not risk free. It doesn't have to be risk free to be vastly superior to everything else. It only needs to be vastly superior to everything else, which it is.
Thank you for your comment and what sounds like a mind that could be opening.
For the record, in my youth, I was an anti-nuke fool, just like many who write here. It was because I was uneducated. Then the Chernobyl reactor exploded, offering us the worst case. I began to educate myself to understand the event. Considering it, I changed my mind.
I have considered nuclear energy to be essential to human survival on a decent and sustainable scale since around 1988 or 1989. I consider it the only form of sustainable energy. I've taken a lot of flack for it, but I support nuclear energy because it is the right thing to do. It's about ethics. I couldn't die with a sense of decency if I had kept my mouth shut.
Have a nice evening and day tomorrow. Thanks again for your comment.
1WorldHope
(900 posts)Is it gas and oil people? Coal? I love this planet and my heart breaks everyday to see what we are doing to it.
eppur_se_muova
(37,397 posts)are more than three K-40 half-lives old. In other words, these organisms existed when potassium had about eight times the radioactivity it does today. One might expect resistance to radiation-induced DNA damage to have been an even more important survival trait then, than today.
I can remember the first time I read a statement like this:
I thought it was a little frightening to realilze how often such errors were generated; I had not known about DNA repair enzymes at the time.DNA undergoes major changes as a result of thermal fluctuations: for example, about 5000 purine bases (adenine and guanine) are lost every day from the DNA of each human cell because their N-glycosyl linkages to deoxyribose hydrolyze, a spontaneous reaction called depurination. Similarly, a spontaneous deamination of cytosine to uracil in DNA occurs at a rate of about 100 bases per cell per day (Figure 5-47).
The excerpt is from this textbook; not one from which I learned what little biochemistry I know -- I used even older texts -- but one that might be worth acquiring. Just reading the little bit excerpted at the link added enormously to my awareness of the role of repair enzymes.
NNadir
(34,662 posts)Those of us who have worked on viral diseases recognize that the reason that viruses are able to evolve so quickly, particularly retroviruses, is their failure to have genetic correction capability.
The pharmaceutical industry put out a fair number of protease inhibitors in the fight against AIDS by the early 90's.
By 2000, these mutants had appeared:
D30N: Nelfinavir. (Agouron/Pfizer).
M46I/I47V/I50V: Amprenavir (BMS).
L10R/M46I/L63P/V82T/I84V: Indinavir (Merck)
M46I/L63P/A71V/V82F/I84V: Ritinovir (Abbott).
Saquinavir: G48V/L90M (Roche)
Antiviral Drug Discovery Summitt, March 28-29, 2001 Protein Science (2000) 9: 1898-1904
(I was involved in the production of three of them, and the phase I and II development of a 4th. It was a very exciting time in my career.)
Most of the mutant viral particles produced were not functional, but because a large number of mutants were generated rapidly, only maybe one in a million had to be functional and among those that were generated, if they had the right mutations, they were selected, by the presence of the protease inhibitor to preferentially breed. (In a person with untreated HIV, about 10 billion viral particles are produced every day.) This mutation rate was especially the case in places where there was noncompliance with the dosing regimen, which might happen for any number of reasons.
I would imagine that for early life, the lack of repair mechanisms led to the rapid rise of diversity, and to the extent it was driven by radiation, it sped evolution.
As more complex life evolved, in particular eucaryotic cells, selection did call for repair mechanisms, and consequently the incurred a survival advantage. The idea that repair mechanisms are stimulated by insults, in particular anti-oxidant enzymes, is offered as the theoretical basis for hormesis, a topic that is increasingly discussed, but not quantifiable at a dose response level. If it pans out, it would not be wholly surprising.
Omics investigations will help things along.
I'm currently working on my first gene insertion project, not on the gene itself, but the gene product. As a result my recent professional readings have been involved in the study of genetic diseases. Our omics instruments are making the pathway to success ever clearer.
In a sense, the mRNA vaccines, an absolutely tremendous scientific and technical triumph, were lit by omics studies.
These tools are very powerful, incredible.