Science
Related: About this forumGraphic Account of the Fukushima Tritium Releases Now Underway: Science.
This news item appeared this week in the prominent scientific journal Science referring to the big bogeyman at Fukushima, and the much discussed release of tritiated water which will take place over the next 30 years.
The risks of radioactive wastewater release
Jim Smith , Nigel Marks, AND Tony Irwin Science 5 Oct 2023 Vol 382, Issue 6666 pp. 31-33
I'm logged into my account, and am not sure whether the article is open sourced, but I'll excerpt and produce the graphic promised in the title of this post.
If you're looking for a scare story to elevate your selective attention on a planet that burst into flame this summer because of the accumulation of the dangerous fossil fuel waste carbon dioxide, do not read on, but spend your time in insipid agonizing, along with the fossil fuel salespeople here and elsewhere trying to rebrand fossil fuels as "hydrogen" and other people engaged in the ennui and excuses for perpetuating the use of fossil fuels and fossil fuel dependent unreliable junk, specifically wind and solar.
A Giant Climate Lie: When they're selling hydrogen, what they're really selling is fossil fuels.
The subtitle:
I'm fairly certain that the coal, oil, and gas burned to power computers by people carrying on about the tritium releases at Fukushima will kill more people than radiation at Fukushima, including those released during the natural disaster that destroyed the reactors (and a city) has ever or ever will.
Excerpts from the news item:
The highest-activity radioactive contaminant in the Fukushima wastewater is tritium (3H), in the form of tritiated water (HTO). This molecule cannot be separated from the wastewater because its chemical behavior is the same as that of nonradioactive water. Like other radionuclides [such as natural carbon-14 (14C) and anthropogenic 137Cs], which emit high-energy γ rays and β particles when they decay, tritium can have biological effects on organisms, particularly DNA damage (8). But tritium’s radiotoxicity by ingestion is very low compared to that of these other radionuclides owing to its very weak β emission and relatively short retention time in the body. Therefore, it is common practice for nuclear facilities worldwide to discharge wastewater containing HTO into the sea (see the figure).
The La Hague nuclear facility in France annually discharges ∼10,000 terabecquerels (1 TBq = 1012 Bq; the becquerel is an amount of a radioactive element that produces one decay per second) of HTO into the English Channel, with annual discharges during 1996–2016 ranging between 8000 and 12,000 TBq (9). Radiation doses to people (measured in sieverts and defined as the amount of energy deposited in tissue, taking account of the response of human biology to different radiation types) from the release of HTO from La Hague are low [less than 0.01 microsieverts per year (μSv/year) (9), which compares with the 1000 μSv/year recommended limit for members of the public from nuclear site releases (2)], and no environmental impacts have been found or are expected...
I promised a graphic in the title of this post, here it is:
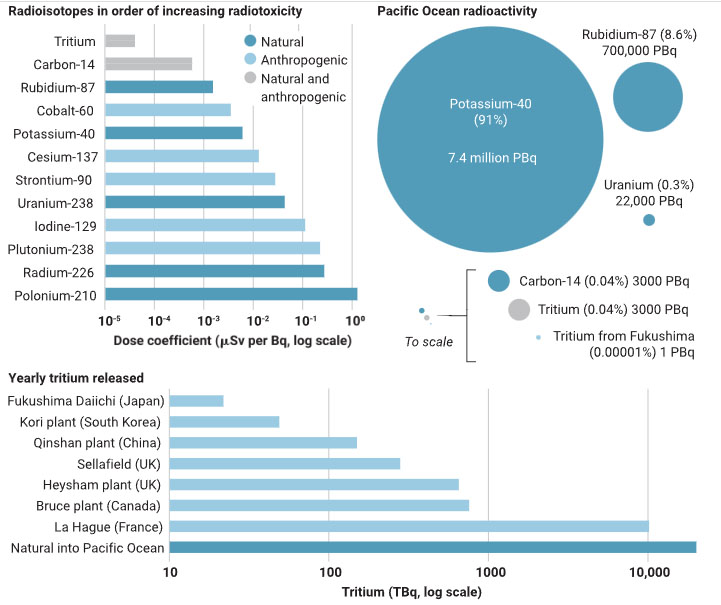
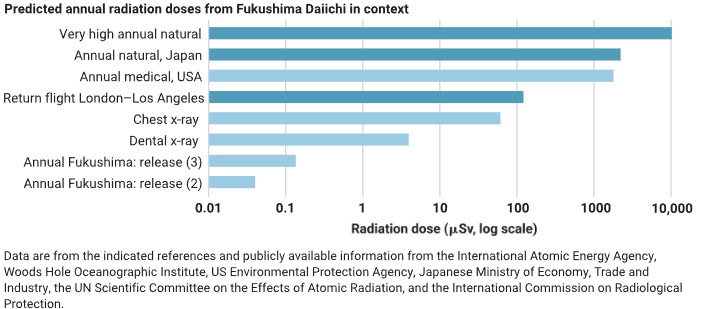
The caption:
Radioisotopes, both naturally occurring and anthropogenic, exhibit different levels of radiotoxicity [based on (15)]. Tritium has a small dose coefficient mainly because its radiation is weak. In the Pacific Ocean, tritium contributes far less total radioactivity than naturally occurring radioisotopes. The planned annual release of tritium from Fukushima Daiichi is much lower than many releases from elsewhere, and the predicted annual radiation doses to local seafood consumers are much smaller than other sources of radiation.
It demonstrates that the radioactivity of the ocean is dominated by the naturally occurring essential element potassium, followed by the congener of potassium, naturally occurring rubidium (which follows potassium biochemically), and finally, the vast resources of naturally occurring uranium found in ocean water as part of the uranium cycle from the planetary mantle that has existed on this planet since the evolution of oxygen in the atmosphere billions of years ago.
Tritium has a relatively short half life, 12.26 years, and ultimately will come into secular equilibrium with the release rates, at which it is decaying at the same rate as it is added. In thirty years about 18% of the tritium released into the oceans and retained in the tanks not yet emptied will still be present, the rest having decayed to the rare stable isotope helium-3.
The highest environmental concentration of tritium in the environment actually took place before the commercial nuclear industry became serious, in 1963, as a result of atmospheric nuclear testing, when the concentrations were many orders of magnitude than they are now. (If interested, one can look it up.) About 3.4% of that tritium, with which everyone on the planet has lived their lives, their entire lives if born after 1963, still remains in the environment.
A final excerpt from the article:
The authors of this article are Australian nuclear engineers. Nuclear engineers are a class of people who have to put up with this kind of selective attention crap, diverting their attention from what should be their main task, saving what is left to be saved in the world, and restoring what can be restored.
Have a pleasant Sunday.
FBaggins
(27,507 posts)It isn’t appropriate to look at the entire pacific. After all - the Exxon Valdez spill was a tiny fraction of the oil that naturally seeps into the pacific each year… yet was devastating for the local environment.
But a more localized analysis would still tell a similar story (of zero risk from dumping water contaminated solely with tritium)
Looking in the other direction- becquerels are not equal in their health risk… and a bq from tritium is of less risk than almost any other form of radiation. 20 keV vs. 1.3 MeV for Potassium 40.
NNadir
(34,533 posts)...tritium can be expected to rapidly diffuse and be carried by currents. Even if we were to look at simple Fick's law diffusion, we would expect it to rapidly disperse owing to the low molecular weight of HTO, 20 g/mol.
But there is a current.
This was covered by the authors of the paper correcting international paroxysms of media stupidity about the famous "Fukushima Tuna Fish" in PNAS: Evaluation of radiation doses and associated risk from the Fukushima nuclear accident to marine biota and human consumers of seafood Nicholas S. Fisher, Karine Beaugelin-Seiller, Thomas G. Hinton, Zofia Baumann, Daniel J. Madigan, and Jacqueline Garnier-Laplace, PNAS June 3, 2013 110 (26) 10670-10675.
They referred to their original paper on tracking the migration of tuna using the (relatively) short lived radioisotope 134Cs released by the Fukushima event.
I quoted some of the text from this paper expressing the scientist's exasperation with our "...but her emails..." media here:
For my 30,000th post, I'd like to thank DU for inspiring me to expand my knowledge, and of course...
There is no way that the tritium that so terrifies radiation paranoids and their fossil fuel enablers and supporters stays localized, absolutely no way. Even if it did - and again and again and again and again it doesn't - the risk would be trivial and not worth the death toll caused by the use of electrical power generated by dangerous fossil fuels to power computers for morons in the media and elsewhere to carry on about it.
Actually the IEA and Japanese authorities have been examining the detection of tritium outside Fukushima, and the signal, if any, is below to the LOD, limit of detection.
IAEA sees no rise in tritium level near Fukushima Daiichi
Thus I stand by my contention, to repeat, that more people have been killed by the air pollution generated by fossil fuels burned to carry on insipidly about this non issue.
On the second point, the full article makes it clear that the risk associated with different nuclei is different, and it is, in fact, addressed in the graphic. The highest dose coefficients are related to the two naturally occurring radionuclides found in the ocean, radium-226 and polonium-210, both members of the uranium decay series, present in seawater as a function the vast natural uranium resources found in the ocean, some 4.5 billion tons. Note that the units in the upper graphic are log scale, tritium is roughly 500,000 lower in risk per Beq than is polonium-210. It also has a vastly shorter biological half-life.
Martin68
(24,468 posts)and cannot mix with water. Most of the oil sinks to the bottom or floats onshore. As the article explains, the radioactive water very rapidly diffuses and is diluted by ocean water, from which it is virtually indistinguishable in behavior. Currents carry it away and rapidly dilute it to infinitesimal concentrations. Considering the radioactivity level is already vanishingly small, it becomes harmless in an ocean the size of the Pacific. No deceit here, just good science.
Mosby
(17,317 posts)The beta radiation is too weak to penetrate skin.
That's why it's used on expensive watches. I have a little fob attached to a flashlight that contains a vial of tritium.