Science
Related: About this forumThe Effect of Brine on the Radiation Driven Near Complete Destruction of "Forever Chemicals"
The paper to which I'll refer in this post is this one: Promotive Effects of Chloride and Sulfate on the Near-Complete Destruction of Perfluorocarboxylates (PFCAs) in Brine via Hydrogen-tuned 185-nm UV Photolysis: Mechanisms and Kinetics Sitao Liu, Gongde Chen, Qingyang Shi, Jay Gan, Bosen Jin, Yujie Men, and Haizhou Liu Environmental Science & Technology 2024 58 (23), 10347-10356.
I will have only a short time to cover the contents, but I'll do my best.
One of the major environmental problems before humanity - dwarfed only by global heating (about which very little, as to border on nothing is being done) is the accumulation of halogenated pollutants in environmental matrices. The most problematic of these in many ways are the compounds now know colloquially as "forever chemicals" more technically described as "PFAS" or perfluoroalkyl substances. (PFAS also refers to one set of the most famous members of this class, perfluoroalkylsulfonates.)
The compounds are now found in almost all of the water in the world, and in the scientific community there is huge concern about the health implications, since they are measurable in all of the world's people.
I think a great deal about these compounds, and have written about them many times in this space. It seems to me that in the case of drinking water, a possible approach to minimizing their intake is to use solid phase extraction - a process relying on adsorption of the pollutant on specially designed materials - which in the removal of these compounds will result in their concentration of them onto the solid materials. However they are not destroyed in this process.
An ideal solid phase extraction agent will be reusable, that is, they can be regenerated by elution with some chemical treatment, but of course such a treatment will still leave the pollutant intact, albeit in much higher concentrations, which still leaves the problem of either making a dump for them or better, destroying them.
PFAS are very difficult to destroy because they have one of the strongest chemical bonds known, the carbon-fluorine bond. The bond energy is such that breaking the bonds require high energy, this in the form of electromagnetic radiation; regrettably visible light is too low energy to break this bond, UV or more energetic wavelengths are involved.
The authors of this paper refer to a method of doing all of the above, solid phase extraction, followed by destruction of the pollutants (or nearly complete destruction of them.)
I like the paper because of my focus on closed cycles for to remediate what is commonly called "waste," where, I contend, the concept of the existence of "waste" is a reflection of poor thinking, lazy thinking. As a species we are a long way from embracing closed cycles which is rather strange given that the biosphere has evolved closed cycles and has functioned for billions of years because of this property. One "waste" that will become problematic if a serious attempt to save humanity from itself is brine, highly concentrated salt solutions derived from nuclear powered desalination, the most efficient of which would be something I described in this space some time ago: The Energy Required to Supply California's Water with Zero Discharge Supercritical Desalination. I can think of many things to do with salts, including utilizing it as a form of energy storage, but this said, the problem is hardly trivial.
One wonders, since humanity refuses to address global heating except with marketing and posturing, wishful thinking, outright lying and throwing good money after bad, whether people would actually care what damage brines do to the environment; all the world's ecosystems are now being treated as potential sites for industrialization via bad ideas advertised as "green," although they are no such thing, but whether it would be possible to overcome indifference, it seems feasible to do something about brine. The proposal in this paper would represent only a tiny fraction of what is needed to use brine in a sustainable closed cycle.
The authors, happily, discuss solid phase extraction in their introduction:
...Physical separation of PFAS, including ion exchange (IX) (18,19) and membrane filtration, (20−22) have been tested for drinking water treatment, through which PFAS are transferred into a brine waste stream. Consequently, PFAS treatment in brine waste has become a major challenge. For example, the regeneration of IX produces a brine with elevated PFAS and chloride. (19,23) The membrane concentrate is also elevated in salinity and PFAS levels. (24) Direct disposal of the brine poses a high risk of secondary contamination to drinking water. Therefore, there is an urgent need for the destruction of PFAS in brine. However, destroying PFAS in brine is challenging due to the complex water matrix. Existing technologies for aqueous PFAS destruction include electrochemical oxidation, (25,26) plasma treatment, (27) and UVC photolysis (λ = 254 nm), (28) but each has its limitations in brine treatment. Electrochemical PFAS oxidation in brine can produce toxic chlorate and perchlorate byproducts. (26,29) Plasma treatment is energy-intensive, with energy consumption ranging from 380 to 833 kWh/m3 for PFOA treatment...
The authors propose the destruction of PFAS in brine with radiation in the far UV range, 185 nm, for which they use a lamp as the source of radiation. High energy (ionizing) radiation in solution generates free electrons, which mechanistically participate in the defluorination of PFAS. Of course lamps require power to run, and if they are run on thermodynamically degraded electricity, a form of energy that is intrinsically dirty because of this degradation, given that we are doing nothing to address climate change, industrial scale use of this technology would further drive global heating, which is now accelerating at the fastest rate ever observed.
Often when I reflect on approaches to this problem, I consider a better radiation source available more or less cheaply, were it not for a certain kind of absurd advertising that is killing the planet, specifically fission products.
Of all the fission products, the one that most intrigues me, is cesium-137, 137Cs which has a half-life of a little over 30 years. It is in secular equilibrium with a short lived nuclear isomer of barium - 137mBa - which decays with a half-life of about 3 minutes to 137mBa's stable naturally occurring isotope 137Ba, . (Barium has interesting properties for the removal of carbon dioxide, and, in fact, the reduction of carbon dioxide to elemental carbon; I've discussed this here, as well.)
The wavelength of the gamma associated with the decay of cesium-137 - actually from the decay of 137mBa - is 0.662 MeV, corresponding to a wavelength, referring to simple physical laws, of 0.00187 nm, much shorter wavelength than the author's UV lamp, and much more energetic. In aqueous solution such energetic photons would generate, via Compton scattering, a plethora of electrons, and thus be far more efficient - and cheaper and cleaner since no thermodynamically degraded electricity would be involved, only direct nuclear energy - at destroying PFAS.
The high energy density of nuclear energy that makes nuclear energy superior to all other forms of energy from a purely environmental standpoint, means that regrettably we cannot accumulate huge quantities of this valuable isotope quickly. In a world with clean energy, where say around 630 exajoules of energy is produced by nuclear energy each year, only a few hundred tons, around 300 tons of 137Cs would accumulate each year, and these quantities would be undergoing continuous decay approaching asymptotically a maximum level at which it is decaying as fast as it is formed. This would take a few centuries to achieve in a sustainable world, and during these years the new accumulations would be smaller and smaller until becoming vanishingly small.
There is a physical limit to how much can form, a function of a series of coupled differential equations known as the Bateman equations:
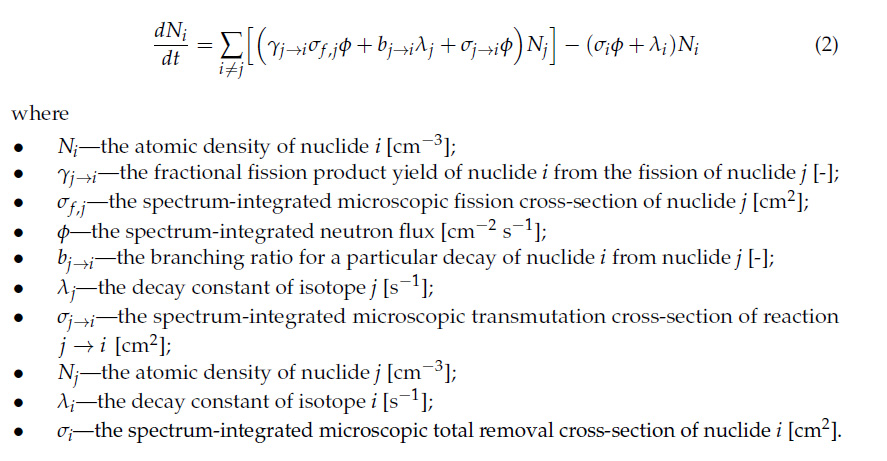
Because 137Cs has a low capture cross section, the terms in the 137Cs equation referring to neutron capture can more or less be ignored.
It can be shown that in a clean sustainable world continuously consuming around 630 Exajoules of nuclear energy, where we had such materials capable of cleaning up messes like PFAS, to a first approximation, it would take around 70 years to have 10,000 tons of 137Cs, with only around 60 new tons accumulating each year.
Since we can only accumulate limited amounts of 137Cs, efficient use of this isotope would require continuous flow systems, exposing as much brine as possible to the radiation from nuclear decay. Such an industrial plant seems feasible to me, particularly in the case where desalination is used to restore the fresh water systems we have so readily and unthinkingly trashed.
In any case, the authors demonstrated excellent destruction of PFAS with their little UV lamp:
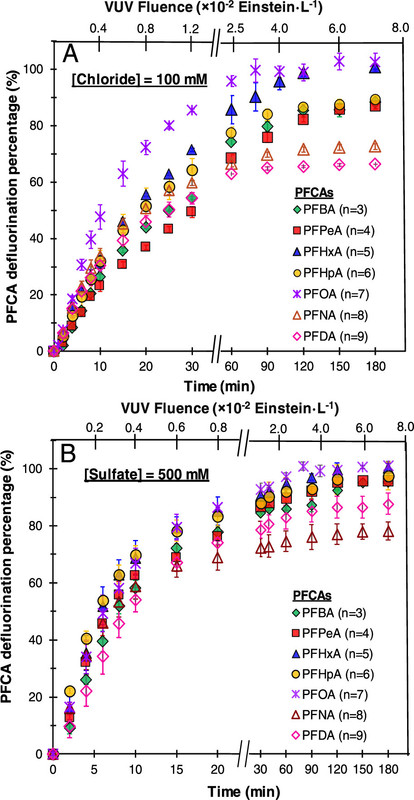
The caption:
They showed that the sulfated brines, sulfate and chloride, help stabilize free electrons making them available for the destruction of PFAS.
It's a nice paper. I like it.
Have a nice weekend.