Science
Related: About this forumRecovery of Fluoride From PFAS "Forever Chemicals" Via Thermal Mineralization.
The paper to which I'll refer in this brief post: Enhancing the Thermal Mineralization of Perfluorooctanesulfonate on Granular Activated Carbon Using Alkali and Alkaline-Earth Metal Additives Charbel Abou-Khalil, Liliya Chernysheva, Anthony Miller, Angela Abarca-Perez, Graham Peaslee, Pierre Herckes, Paul Westerhoff, and Kyle Doudrick Environmental Science & Technology 2024 58 (25), 11162-11174
In 1987 the world's main ores of the mineral cryolite, (Na3AlF6)which was mined mainly in Greenland were depleted. This is a key mineral in the production of aluminum, in an electrochemical process, the Hall process, in which the reaction is carried out in a cryolite molten salt at high temperatures. Happily it is straight forward to synthesize cryolite by treating bauxite with HF and sodium fluoride.
The main ore for fluorine is fluorite, the insoluble salt CaF2. It too, like cryolite may be subject to depletion; to be clear I'm not up to date on this risk, should it be problematic. This said, I am a fan of closed matter cycles.
Much of the world's fluorine has been used industrially to manufacture PFAS "perfluoralkylated substances" which have emerged as serious intractable pollutants as I noted recently in another post.
The Effect of Brine on the Radiation Driven Near Complete Destruction of "Forever Chemicals"
This paper refers to a scheme to recover fluorine by "mineralizing" (destroying) PFAS thermally.
The authors here note that one solid phase adsorbent for the removal of PFAS is granulated activated carbon, but as discussed in the previous post this process removes PFAS, but does not destroy them.
From the introduction:
Conventional water treatment processes face limitations in effectively removing and destroying PFAS. (15,16) Adsorbents such as granular activated carbon (GAC) are an attractive prospect for removing PFAS from water due to the low cost. (16) However, managing spent adsorbents contaminated with PFAS poses a disposal challenge. The United States Environmental Protection Agency and the Department of Defense (DoD) have outlined interim guidance for handling PFAS-laden solids, suggesting landfilling, thermal reactivation, and incineration as potential methods. (17,18) Landfilling carries the risk of PFAS re-entering the environment, (19) making thermal treatment more practical for end-of-life destruction or regeneration of PFAS-contaminated GAC. (20−23) Yet, the thermal destruction of PFAS requires very high temperatures (>1000 °C) and it releases undesirable products of incomplete destruction (PIDs) in the flue gas. (15,24−26) As a result, the DoD has advised exercising caution in employing thermal treatment for managing PFAS wastes until further information is acquired. (17)
The authors study the use of various basic mineral bases to overcome these limitations. One of the byproducts of the thermal decomposition of PFAS is HF gas, which is an extremely valuable but extremely dangerous, as it is highly toxic and corrosive, industrial reagent. (It's main use is the cracking of petroleum to make gasoline.) HF is one product of the thermal decomposition of PFAS.
(For the record, I favor the HF molten salt procedure for the recovery of valuable components of used nuclear fuels.)
The authors examine various procedures for minimizing by products like HF using inorganic oxides.
The authors find, unsurprisingly, that calcium hydroxide yields the best results, generating a synthetic form of the mineral fluorite.
A figure from the paper:
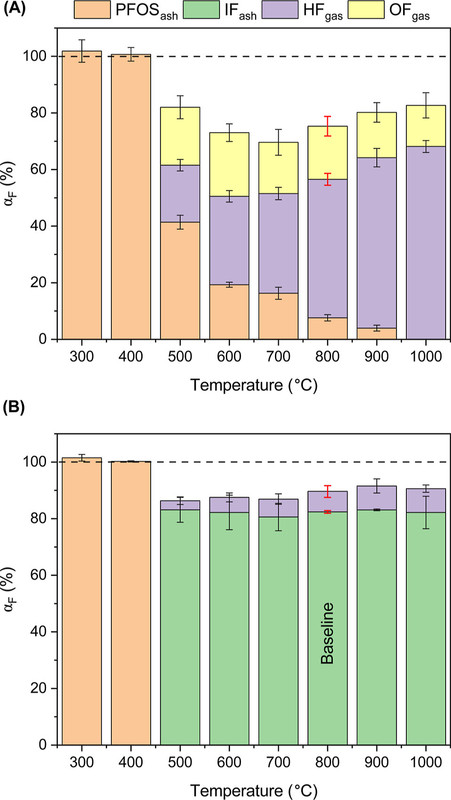
The caption:
"IF" refers to inorganic fluorides, "OF" to organic fluorides, probably mostly TFA, trifluoroacetic acid, although they do not identify this as such.
They evaluate the effect of Ca/F ratios on the results:
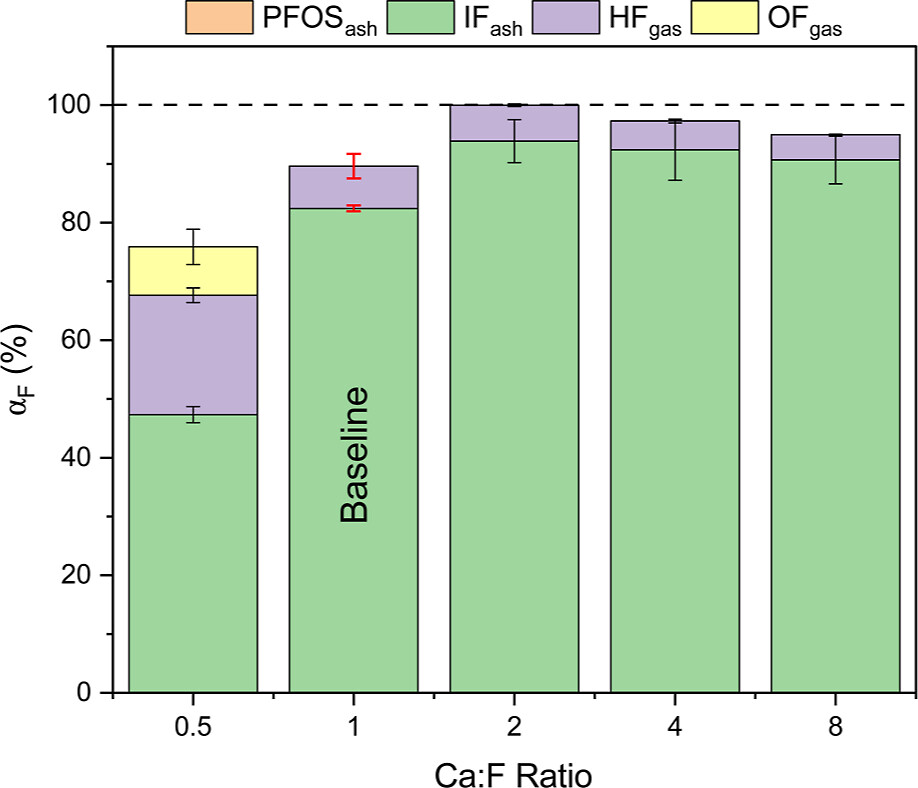
The caption:
From the paper's conclusions:
In the rest of the conclusion the authors note some limitations and areas of additional study.
This is a nice paper from the annals of study of closed matter cycles.
I like it.
Have a nice day tomorrow.