Welcome to DU!
The truly grassroots left-of-center political community where regular people, not algorithms, drive the discussions and set the standards.
Join the community:
Create a free account
Support DU (and get rid of ads!):
Become a Star Member
Latest Breaking News
Editorials & Other Articles
General Discussion
The DU Lounge
All Forums
Issue Forums
Culture Forums
Alliance Forums
Region Forums
Support Forums
Help & Search
Science
Related: About this forumResearchers discover a surprising way to jump-start battery performance
https://www6.slac.stanford.edu/news/2024-08-29-researchers-discover-surprising-way-jump-start-battery-performanceAUGUST 29, 2024
Researchers discover a surprising way to jump-start battery performance
Charging lithium-ion batteries at high currents just before they leave the factory is 30 times faster and increases battery lifespans by 50%, according to a study at the SLAC-Stanford Battery Center.
By Glennda Chui
A lithium-ion battery’s very first charge is more momentous than it sounds. It determines how well and how long the battery will work from then on – in particular, how many cycles of charging and discharging it can handle before deteriorating.
In a study published today in Joule, researchers at the SLAC-Stanford Battery Center report that giving batteries this first charge at unusually high currents increased their average lifespan by 50% while decreasing the initial charging time from 10 hours to just 20 minutes.
Just as important, the researchers were able to use scientific machine learning to pinpoint specific changes in the battery electrodes that account for this increase in lifespan and performance – invaluable insights for battery manufacturers looking to streamline their processes and improve their products.
The study was carried out by a SLAC/Stanford team led by Professor Will Chueh in collaboration with researchers from the Toyota Research Institute (TRI), the Massachusetts Institute of Technology and the University of Washington. It is part of SLAC's sustainability research and a broader effort to reimagine our energy future leveraging the lab’s unique tools and expertise and partnerships with industry.
[...]
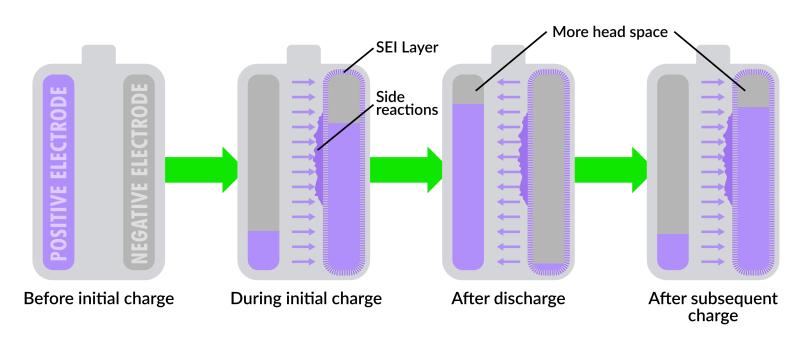
Factory-charging a new lithium-ion battery with high currents significantly depletes its lithium supply but prolongs the battery’s life, according to research at the SLAC-Stanford Battery Center. The lost lithium is generally usually used to form a protective layer called SEI on the negative electrode. However, under fast charging conditions, lithium ions are also consumed during side reactions at the negative electrode. This creates additional headspace in both electrodes and helps improve battery performance and lifespan.
[...]
Researchers discover a surprising way to jump-start battery performance
Charging lithium-ion batteries at high currents just before they leave the factory is 30 times faster and increases battery lifespans by 50%, according to a study at the SLAC-Stanford Battery Center.
By Glennda Chui
A lithium-ion battery’s very first charge is more momentous than it sounds. It determines how well and how long the battery will work from then on – in particular, how many cycles of charging and discharging it can handle before deteriorating.
In a study published today in Joule, researchers at the SLAC-Stanford Battery Center report that giving batteries this first charge at unusually high currents increased their average lifespan by 50% while decreasing the initial charging time from 10 hours to just 20 minutes.
Just as important, the researchers were able to use scientific machine learning to pinpoint specific changes in the battery electrodes that account for this increase in lifespan and performance – invaluable insights for battery manufacturers looking to streamline their processes and improve their products.
The study was carried out by a SLAC/Stanford team led by Professor Will Chueh in collaboration with researchers from the Toyota Research Institute (TRI), the Massachusetts Institute of Technology and the University of Washington. It is part of SLAC's sustainability research and a broader effort to reimagine our energy future leveraging the lab’s unique tools and expertise and partnerships with industry.
[...]

Factory-charging a new lithium-ion battery with high currents significantly depletes its lithium supply but prolongs the battery’s life, according to research at the SLAC-Stanford Battery Center. The lost lithium is generally usually used to form a protective layer called SEI on the negative electrode. However, under fast charging conditions, lithium ions are also consumed during side reactions at the negative electrode. This creates additional headspace in both electrodes and helps improve battery performance and lifespan.
[...]
=================
Joule:
https://www.cell.com/joule/abstract/S2542-4351(24)00353-2
(limited access)
August 29, 2024
Data-driven analysis of battery formation reveals the role of electrode utilization in extending cycle life
Xiao Cui, Stephen Dongmin Kang, Sunny Wang, Justin A. Rose, Huada Lian, Alexis Geslin, Steven B. Torrisi, Martin Z. Bazant, Shijing Sun, William C. Chueh
Context & scale
High-performance and low-cost Li-ion batteries are crucial for electrifying transportation and deepening the penetration of renewables in the electricity grid. However, a manufacturing step known as formation bottlenecks the throughput. Many fast formation protocols have been proposed to decrease formation time without compromising battery performance, but we lack a generalized understanding of how formation parameters affect battery cycle life.
In this work, we employ data-driven workflows to efficiently explore and understand the formation parameter space. We identify two key parameters—formation charge current and temperature—and demonstrate their distinct impact on the aging mechanisms. Specifically, we show how fast formation extends battery cycle life by shifting the electrode-specific utilization range. The mechanisms revealed by our study can be generalized to optimize formation protocols and design optimal battery operational ranges.
Highlights
[..]
Data-driven analysis of battery formation reveals the role of electrode utilization in extending cycle life
Xiao Cui, Stephen Dongmin Kang, Sunny Wang, Justin A. Rose, Huada Lian, Alexis Geslin, Steven B. Torrisi, Martin Z. Bazant, Shijing Sun, William C. Chueh
Context & scale
High-performance and low-cost Li-ion batteries are crucial for electrifying transportation and deepening the penetration of renewables in the electricity grid. However, a manufacturing step known as formation bottlenecks the throughput. Many fast formation protocols have been proposed to decrease formation time without compromising battery performance, but we lack a generalized understanding of how formation parameters affect battery cycle life.
In this work, we employ data-driven workflows to efficiently explore and understand the formation parameter space. We identify two key parameters—formation charge current and temperature—and demonstrate their distinct impact on the aging mechanisms. Specifically, we show how fast formation extends battery cycle life by shifting the electrode-specific utilization range. The mechanisms revealed by our study can be generalized to optimize formation protocols and design optimal battery operational ranges.
Highlights
• 186 Li-ion batteries cycled across 62 formation protocols but with the same aging test
• High-formation charge current on the first cycle extends battery cycle life by up to 70%
• Substantial Li loss during fast formation shifts the electrode utilization range
[..]
1 replies
 = new reply since forum marked as read
Highlight:
NoneDon't highlight anything
5 newestHighlight 5 most recent replies
= new reply since forum marked as read
Highlight:
NoneDon't highlight anything
5 newestHighlight 5 most recent replies
Researchers discover a surprising way to jump-start battery performance (Original Post)
sl8
Sep 2024
OP
Fast-charging at the start and extending battery life by 50% sounds like a huge step forward. If they can use this method to improve batteries for everyday use, that’d be amazing for everything from phones to EVs.