It seems the egg came before the chicken.
This came up in one of my news feeds: What Came First the Chicken or the Egg? An Ancient Unicellular Indicates It Was the Egg
Subtitle:
Research suggests that nature would have possessed the genetic tools to “create eggs” long before it “invented chickens”.
An excerpt of the news feed item:
Chromosphaera perkinsii is a single-celled species discovered in 2017 in marine sediments around Hawaii. The first signs of its presence on Earth have been dated at over a billion years, well before the appearance of the first animals. A team from the University of Geneva (UNIGE) has observed that this species forms multicellular structures that bear striking similarities to animal embryos. These observations suggest that the genetic programs responsible for embryonic development were already present before the emergence of animal life, or that C. perkinsii evolved independently to develop similar processes. Nature would therefore have possessed the genetic tools to “create eggs” long before it “invented chickens”. This study is published in the journal Nature.
The first life forms to appear on Earth were unicellular, i.e. composed of a single cell, such as yeast or bacteria. Later, animals - multicellular organisms - evolved, developing from a single cell, the egg cell, to form complex beings. This embryonic development follows precise stages that are remarkably similar between animal species and could date back to a period well before the appearance of animals. However, the transition from unicellular species to multicellular organisms is still very poorly understood...
The full
Nature article is here:
Olivetta, M., Bhickta, C., Chiaruttini, N. et al.
A multicellular developmental program in a close animal relative. Nature 635, 382–389 (2024).
The introductory text from the full paper:
The evolution of multicellular organisms from their unicellular ancestors marks a major transition in the history of life on Earth5,6,7. This transition was accompanied by fundamental developmental challenges such as generating diverse cell types, forming three-dimensional tissues and establishing overall coordination to drive body plan formation. Asymmetric cell division contributes to cellular diversity8,9, and the formation of three-dimensional tissues relies on the precise coordination of cell divisions, adhesion and signalling10,11,12. Compared to other eukaryotic lineages, animal multicellularity stands out by relying on an autonomous developmental program, predominantly driven by intrinsic signals, that drives the emergence of an extensive variety of cell types from a single-celled zygote13,14,15. Although some processes of embryogenesis are found in plants, fungi and various algae, the sheer diversity of specialized cell types in animals highlights a unique aspect of their evolutionary trajectory. Throughout early animal embryogenesis, several ordered processes take place, including cleavage divisions, axis establishment, zygotic genome activation and spatial organization of germ layers. This sequence, although it displays remarkable conservation across species, exhibits a degree of developmental plasticity, highlighting the adaptability of development to distinct ecological pressures1,16. Despite this plasticity, the fundamental aspects of this developmental sequence indicate that parts of the underlying program may have originated before the emergence of animals themselves17. One possibility is that embryogenesis represents an animal innovation; alternatively, it might have evolved from pre-existing developmental processes present in the last common unicellular ancestor. Animals are closely related to choanoflagellates, filastereans, pluriformeans and ichthyosporeans (Fig. 1a)4. These lineages not only partly share a genetic toolkit used by animals for development but also can form transient multicellular structures and display various temporary cell stages in distinct environments5. Current evidence indicates that the formation of clonal multicellular choanoflagellate colonies18,19,20 and the emergence of filastereans aggregates21,22,23, both morphologically distinct from any known embryonic stages from living animals and their fossil relatives24, occur as a facultative response to external chemical cues18,19,25,26,27,28...
The authors study an single cell organism that undergoes palintomic cell division, which is cell division without the cluster of newly formed cells being larger than the original cell, as is the case in the very early phases of embryo development:
The organism is
Chromosphaera perkinsii, a single celled organism that is thought to bear close relationship to early animal species. It is in a class of organisms known as dermocystids, part of a clade of eucaryotic cells, Ichthyosporea, with two branches, Ichthyophonida and Dermocystida which are generally parasites on amphibians, but in this case, the organism is free living.
The way these cells behave is quite interesting:
...Among close animal relatives, the Ichthyosporea, comprising two primary lineages, Dermocystida and Ichthyophonida (Fig. 1a)32,33, exhibit a variety of life cycles that combine fungal-like characteristics with transient multicellular stages reminiscent of early animal development33. Most ichthyophonids, including the model Sphaeroforma arctica, undergo coenocytic development, characterized by synchronized nuclear divisions without cytokinesis34,35,36,37. This process depends on an acentriolar microtubule organizing centre that drives a fungal-like closed mitosis38. On reaching a specific nuclear-to-cytoplasmic ratio39, S. arctica undergoes an actomyosin-dependent cellularization. During this process, a transient multicellular layer resembling an animal epithelium is formed before the release of new-born cells to repeat the cycle36. In contrast, we recently showed that C. perkinsii, the only cultured free-living dermocystid3, undergoes centriole-mediated, animal-like open mitosis coupled with cleavage divisions38. This discovery indicates that C. perkinsii proliferates through a palintomic developmental mode, highlighting the vast range of cellular and developmental diversity in the Ichthyosporea. However, nothing is known at present about the spatiotemporal dynamics of the ichthyosporean palintomic life cycle, nor is it clear whether it is orchestrated by an autonomous developmental program that exhibits any similarities with early animal embryogenesis...
A figure from the text:
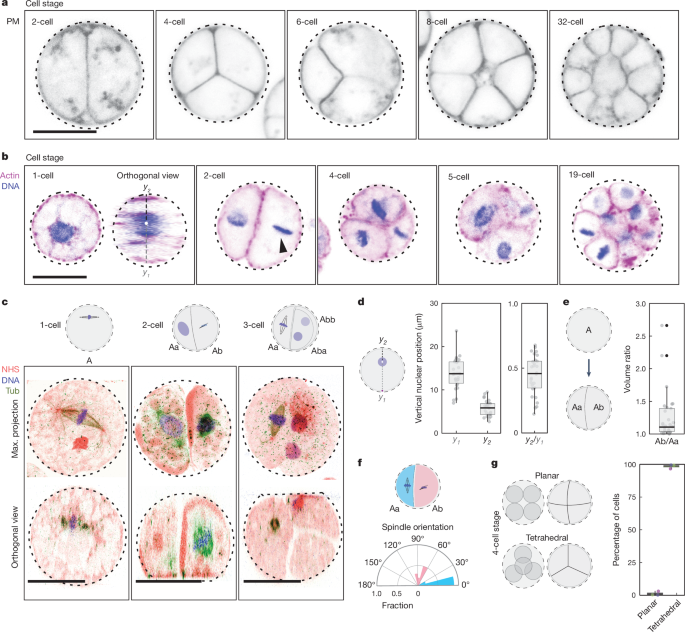
The caption:
a, Plasma membrane-stained (PM) live colonies at distinct cell stages, highlighting the patterned cleavage divisions, tetrahedral four-cell stage and formation of spatially organized multicellular colonies (Supplementary Video 5). b, Actin- (magenta) and DNA-stained (blue) colonies at distinct cell stages showcasing nuclear cortical positioning, asymmetrical cell division (in volume and in time) and the formation of a multicellular colony. This result has been reproduced at least three independent times. c, U-ExM stained colonies for pan-labelling with NHS-Ester (red), microtubules (green) and DNA (blue), highlighting the first mitotic division at the cortex, perpendicular spindles at the two-cell stage and a three-cell stage (Supplementary Video 6). d, Box plot showing the vertical localization of the nucleus at the one-cell stage before or at the first cleavage division highlighting the nuclear cortical localization (n = 28 cells). Box plots extend from the 25th to 75th percentile, including the median, and whiskers extend from the minimum to maximum value. e, Box plot illustrating the volumetric ratio following the first division resulting in Ab and Aa cells, highlighting the asymmetrical cell division (n = 28 two-cell-stage cells). The volume ratio is >1 (one-sided Wilcoxon signed-rank test, P = 3.73 × 10−9). Box plots extend from the 25th to 75th percentile, including the median, and whiskers extend from the minimum to maximum value. f, A polar plot representing the spindle angle for the Aa and Ab cells during mitosis demonstrates that the Ab cells divide perpendicular to the first cleavage division, whereas the Aa cells divide parallel to it (n = 11 colonies). g, Box plot showing the percent of the four-cell stage exhibiting a planar or tetrahedral spatial organization (n = 108 colonies). Box plots extend from the 25th to 75th percentile, including the median, and whiskers extend from the minimum to maximum value. Scale bars, 10 µm. Max., maximum; tub, microtubules.
What is interesting is that the cells are
differentiated on division:
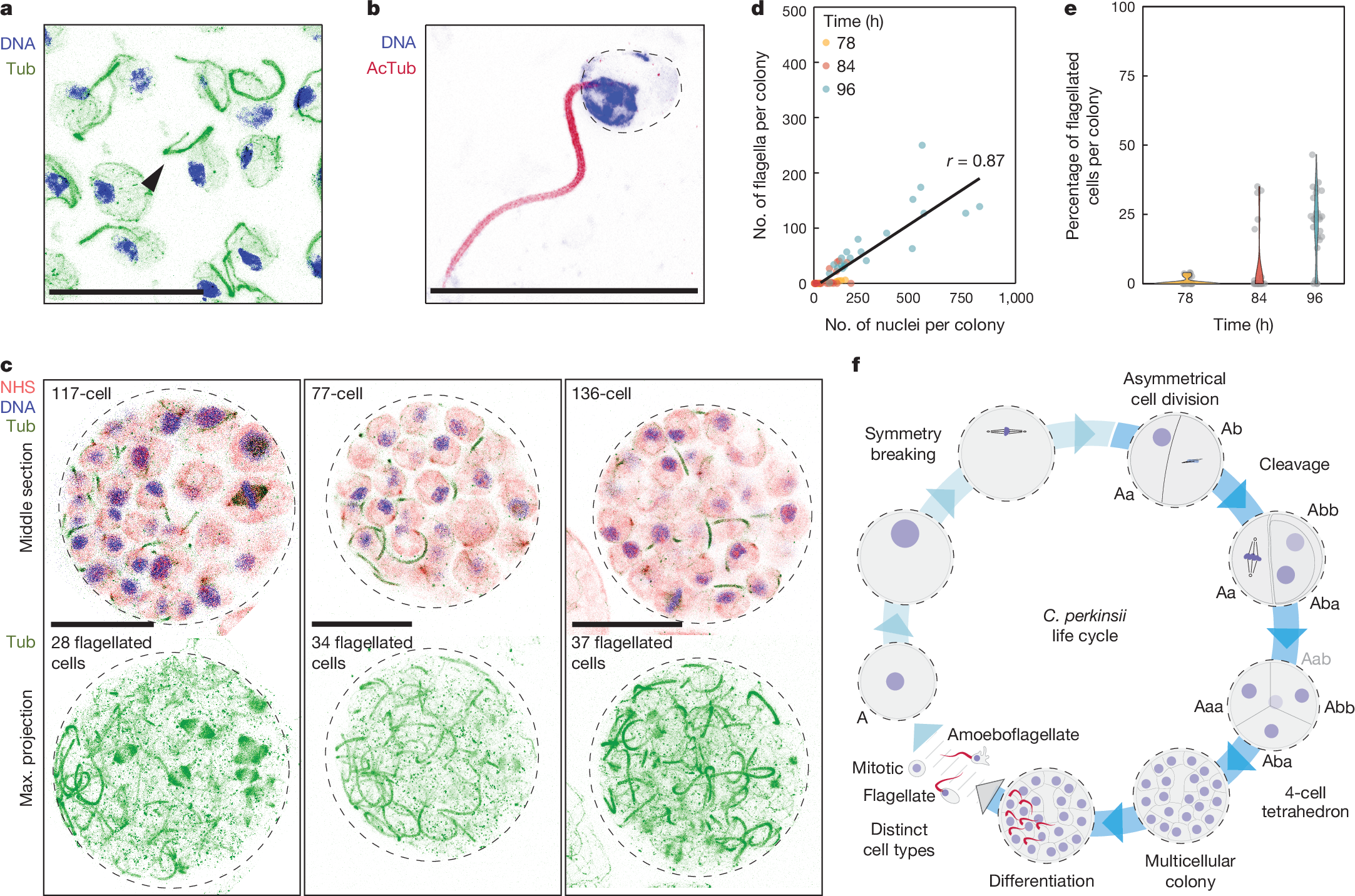
a, U-ExM stained cells for microtubules (green) and DNA (blue), identifying flagellated cells (arrow). This result has been reproduced at least three independent times. b, U-ExM stained flagellated cell for acetylated tubulin (AcTub; dark red) and DNA (blue). This result has been reproduced at least three independent times. c, U-ExM stained late colonies for pan-labelling with NHS-Ester (red), microtubules (green) and DNA (blue), highlighting the co-existence of flagellated and non-flagellated cells in the multicellular colony (Supplementary Videos 9 and 10). d, A dot plot illustrating the number of flagellated cells per colony, which increases with the overall number of cells per colony. Notably, although there is a positive correlation, the increase is not linear, showing that distinct cell types coexist in the multicellular colonies (n78 = 33, n84 = 32, n96 = 27 colonies per time point). e, Violin plot showing the percent of flagellated cells in a multicellular colony at distinct developmental times (n78 = 33, n84 = 32, n96 = 27 colonies per time point). f, A model representing the developmental program of C. perkinsii, beginning with the relocation of the nucleus to the cortex, symmetry breaking at the 1- to 2-cell stage, formation of a tetrahedral four-cell stage, development into a multicellular colony with intricate three-dimensional architecture, differentiation of a subset of cells into flagellated cells and release of distinct cell types. Scale bars, 10 µm.
The authors conclude:
Exploring the evolutionary history that led to the emergence of animal multicellularity has been challenging. This stems notably from the paucity of non-metazoan holozoans fossils and the limitations stemming from studying only a few closely related protists, primarily focusing on their genomic content and often neglecting their cellular physiology5,6,51. Here we characterize the life cycle of the ichthyosporean C. perkinsii, a close animal relative, providing evidence for an intrinsic and clonal multicellular developmental program. Our results show that C. perkinsii undergoes palintomic development (Fig. 4f), involving cleavage divisions coupled to open mitosis, diverging from the coenocytic development and cellularization in other studied ichthyosporeans37. The observation that C. perkinsii positions the nucleus cortically in the one-cell stage, undergoes asymmetric cell division, forms organized multicellular colonies and spatially differentiates into distinct cell types demonstrates key morphological and transcriptional parallels with the early stages of animal embryogenesis1. Notably, the development of C. perkinsii is stereotypical and occurs independently of apparent external triggers, leading to the formation of colonies harbouring hundreds of cells (Fig. 4f). Such capacity shows that an autonomous cell specification program with seemingly spatial cell differentiation has evolved in C. perkinsii, a developmental trait considered as a hallmark of ‘complex’ multicellular lineages such as animals, land plants, red and brown algae and mushroom-forming fungi7...
Interesting, I think.

