Science
Related: About this forumIn Source Fragmentation of PFAS in Mass Spectrometry Complicates the Identification of These Ubiquitous Pollutants.
The paper to which I'll briefly refer in this post is this one: Unravel the in-Source Fragmentation Patterns of Per- and Polyfluoroalkyl Substances during Analysis by LC-ESI-HRMS Ke Wang, Runyun Wang, Wenyu Shan, Zilin Yang, Yinjuan Chen, Lei Wang, and Yanyan Zhang Environmental Science & Technology 2024 58 (51), 22766-22776.
One of the major environmental problems before the world, which faces vast problems, the most dire of which is the collapse of the planetary atmosphere, is the problem of PFAS (Per and Poly Fluorinated Substances) caused by the widespread use of these compounds in many technologies and products, the most famous of which is Teflon, but hardly limited to it. Technologies responsible for this pollution include fire fighting foams, hydrogen fuel cells, lubricants and other members of a long list of compounds.
Often attempts to replace these compounds with others results in new compounds that are as bad, or even worse, than the compound they were designed to replace.
A big problem is that there are literally thousands of PFAS molecules, and the paper is useful for giving some insight, albeit limited, to the diversity of these compounds, complicated by their degradation pathways in the environment which leads to other, potentially toxic, unknown products.
The paper is somewhat esoteric and concerns the most powerful analytical technique in chemistry in my opinion, mass spectrometry, and represents, for lack of a better term, a Heisenbergian case, where looking at the system results in changes to the system that may complicate the observation.
From the text:
Although only molecular ions are desired, in-source fragmentation (ISF) inevitably occurs even in a soft ESI source and generates fragment ions. (13) These ISF ions coelute with molecular ions, and failure to distinguish them can lead to misannotation of MS features. (14) This increases data complexity and uncertainty during nontarget analysis and transformation product identification, undermining the credibility of newly identified structures and potentially resulting in misinterpretation of degradation mechanisms. (13) The negative impacts of ISF have been well-recognized in omics analysis of biological samples. (12-14) Nevertheless, the ISF patterns of analytes resemble those of CID in tandem MS. (13,15) Thus, ISF is often used to provide pseudo-MS2 spectra, enabling the acquisition of additional structural information during nontarget analysis. (16,17) In practice, ISF features are primarily identified based on the coelution pattern with molecular ions, their presence in the MS2 spectra of molecular ions, and their similar fragmentation patterns to those of CID. (13)
ISF of PFAS has been noted in recent studies but has yet to be systematically characterized in LC-ESI-HRMS. Strong ISF has been observed for perfluoroalkyl ether carboxylates (PFECA) at the weak ether group with relatively low bond dissociation energies (BDE), which helped the discovery of new compounds but also reduces or even eliminates the signal intensity of molecular ions, resulting in poor sensitivity during quantitative analysis. (18-20) ISF has successfully facilitated the identification of new PFAS classes, including chlorine (Cl-PFCA, ClCnF2nCO2–) or hydro-substituted perfluorocarboxylates (H-PFCA, HCnF2nCO2– ). (17) For Cl-PFCA, the elimination of Cl– and the neutral loss of CO2 ([M-44 ]- ) occur in ESI, whereas the neutral loss of CO2HF ([M–64]− ) is observed for H-PFCA. (17) In a few studies, ISF ions from neutral loss of CO2 and/or HF were found in the full scan MS1 spectra of carboxylic PFAS, though they were not explicitly mentioned. (21-23) Coelution correlation has been proposed for use in finding ISF ions in some newly developed nontarget screening tools. (24)
The inadvertent oversight of ISF has led to the misannotation of PFAS structures during nontarget analysis and transformation product identification. For instance, previously identified unsaturated perfluorinated alcohols were suspected to be ISF ions of PFCA (CnF2n+1CO2–) following a rearrangement process in the ion source. (25,26) ISF ions (CnF2n+1–, [M-44 ]- ) resulting from neutral loss of CO2 were erroneously recognized as degradation products of perfluorooctanoic acid (PFOA), undermining the reliability of the proposed degradation pathways. (27)...
...Herein, we comprehensively analyzed the ISF potentials of 82 PFAS in 12 distinct classes using LC-ESI-HRMS, aiming to represent a significant portion of the structures found in the environment. We identified the PFAS classes and structure characteristics that are susceptible to ISF and proposed seven ISF pathways...
To give an idea of the variability of these compounds - note that the are representatives of classes of compounds - one can look at this figure from the paper:
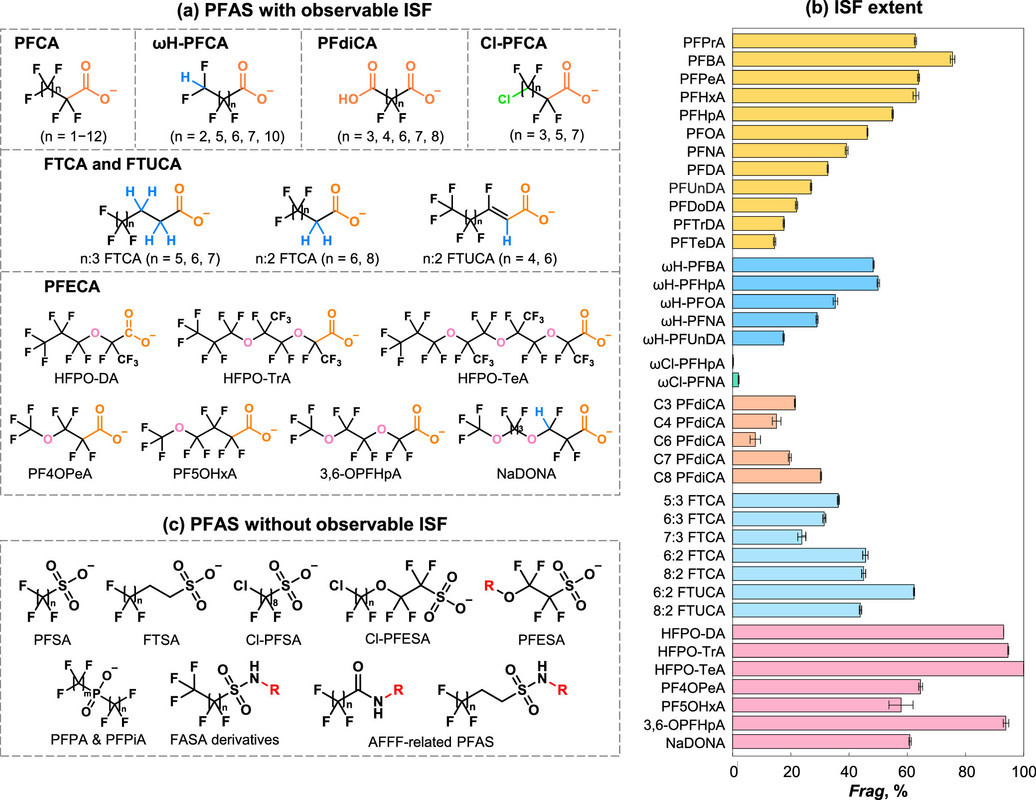
The caption:
Note that the perfluoroethers were designed to ameliorate the risks of the straight chain perfluorinated substances, but it is recognized that they are probably problematic in their own right, although they may degrades somewhat more easily.
A somewhat technical discussion of the fragmentation mechanisms of in source fragmentation:
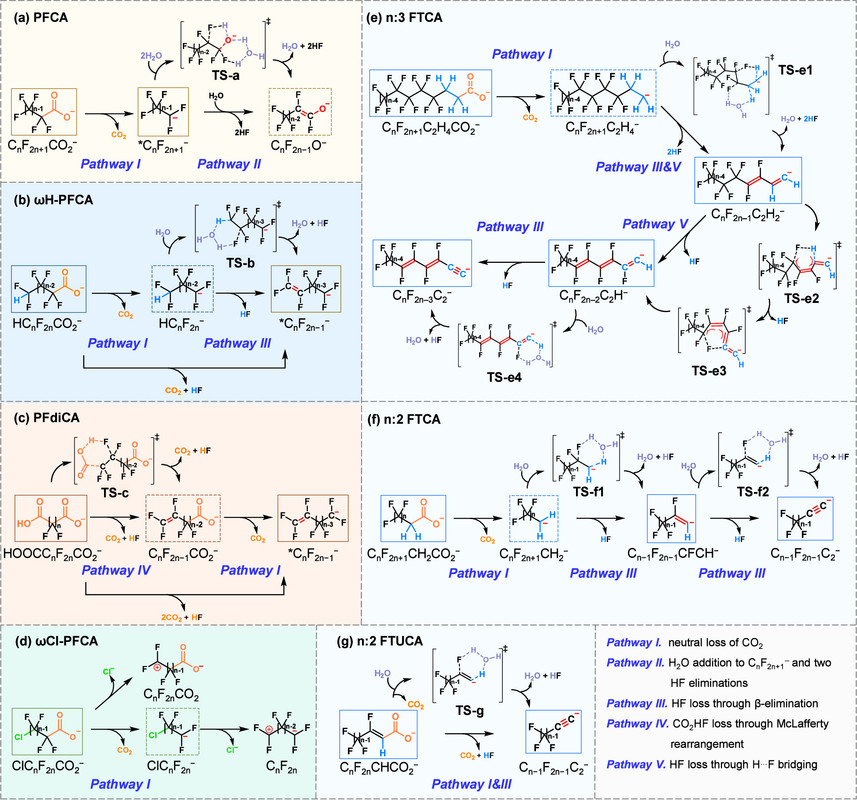
A technical listing of adjustments to parameters in the mass spectrometer and the behavior of the molecules. (Note that the instrument used in the paper was a low level Orbitrap, an Exploris 120, and not one of the more sophisticated mass spectrometers now available.)
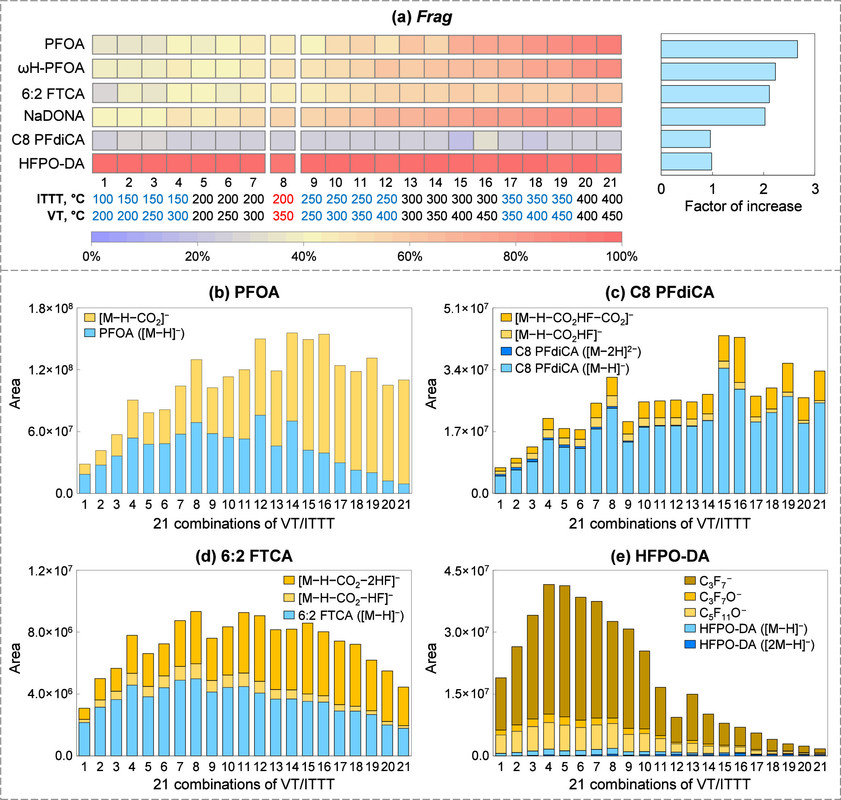
This paper strikes me as important.
I was amused some time ago by an antinuke here - now residing happily on my ignore list - remarked that I am subject to the "if your only tool is a hammer, every problem is a nail" cliche, which is accurate. Most of the environmental solutions that occur to me involve radiation. I propose that the ultimate destruction of PFAS, perhaps after adsorptive removal and regeneration of solid phase adsorbents so utilized, is radiolytic, using fission products as a source of radiation in basic solutions in a Brayton cycle nuclear power plant with air as the working fluid. This process will necessarily involve many intermediates before mineralization to fluoride salts and carbon dioxide, and it will be important to track these, especially given the wide range of compounds involved.
Regrettably, we do not as of now have enough radioactive materials to make much of a dent in these pathways, and air based Brayton cycles for nuclear reactors are not under design anywhere to my knowledge, but I've told my kid about it, and members of the faculty, post docs and grad students at his institutions with whom he's raised the point of fission product driven degradation of PFAS have, as I've understood it, universally understood that it's a good idea.
Exposure of the air to radiation fields may serve a similar advantage.
Have a happy New Year.