Science
Related: About this forumHighly Efficient Plutonium Extraction Using a Phosphonium Phosphinate Task-Specific Ionic Liquid
The paper to which I'll refer in this post is this one: Highly Efficient Plutonium Extraction from Nitric Acid Feeds Using a Phosphonium Phosphinate Task-Specific Ionic Liquid Surekha D. Chowta, Arijit Sengupta, and Prasanta K. Mohapatra Industrial & Engineering Chemistry Research 2025 64 (2), 1252-1264.
The paper was published by Indian scientists.
As an advocate of the expanding use of nuclear energy, it behooves me to note, as India is a nation operating PHWRs, pressurized heavy water reactors, which as is the case with Canada, where the reactors are known as CANDUs, that dominate their nuclear power fleet, that this paper, does have nuclear weapons proliferation value, as CANDU type reactors allow for operando refueling. This said, nuclear weapons seem to have the advantage that people who possess them are generally afraid to use them. This said, there has always been a risk of the weapons falling into the hands of some of the most awful people on the planet, such as we have to our surprise, observed in the United States, where the slime mold in the White House can definitely be characterized as a "most awful person." Operando fuel replacement does allow for the preparation of weapons grade plutonium, although it can also be used to produce denatured plutonium not suitable for use in nuclear weapons, in fact, instantaneous denaturation by making reactor grade plutonium readily available in pure form.
Nevertheless, despite its risk of destroying the world, plutonium, in my view - this is unchanged despite the aforementioned caveat - essential to have any hope of saving the world and possibly even restoring what is possible to restore. Thus, risks involving easily observed human stupidity notwithstanding, this paper has much to recommend it.
From the paper's introduction:
Ionic liquids have been termed by some as “green” diluents, as they can be potential alternatives to the volatile organic compounds (VOCs), i.e., the molecular diluents due to favorable characteristics like low vapor pressure, high degree of thermal, chemical, and radiation stabilities, a wide range of electrochemical window, high solubility of several inorganic, organic, and polymeric materials, etc. (1−8) In nuclear fuel cycle activities, molecular diluents have been used in solvent extraction processes. However, lab-scale studies with ionic liquids have shown promise with much enhanced metal ion extraction. (9−12) The most attractive property of an ionic liquid diluent is the latter’s high degree of tunability. The metal ion extraction by n-butyl-substituted methyl imidazolium ionic liquids exhibited a “cation exchange” mechanism involving cationic species, while in the case of higher homologue, i.e., n-octyl-substituted methyl imidazolium-based ionic liquids, the extraction was found to predominantly proceed via a “solvation” mechanism involving neutral species. (13−16) Tuning of the extraction mechanism from “cation exchange” to “solvation” to “anion exchange” was reported to be successful, employing different variants of the ionic liquids, i.e., methyl imidazolium, pyridinium, and pyrrolidinium for the extraction of the uranyl ion using the same amide-based ligands. (17) Using appropriate combination of cations and anions of ionic liquids; the viscosity of the same can be modified and hence, the extraction kinetics can be tuned. (18−20) A proper understanding of the structure–activity relationship was found to be useful in fine-tuning the desired properties of the ionic liquids by minute structural modifications. (21,22) While the imidazolium- and pyridinium-based ionic liquids are often studied by researchers, the phosphonium-based ionic liquids are not well studied, though some of those are commercially available.
Nonaqueous processing techniques, frequently based on molten salts or ionic liquids, are capable of minimizing the volume of radioactive liquid wastes; while also mitigating corrosion issues arising in aqueous systems. Additionally, these methods are less susceptible to the generation of hydrogen gas, which is a safety hazard in aqueous reprocessing. The ability to achieve a more efficient separation of valuable materials such as uranium, plutonium, and minor actinides represents another significant advantage, as it promotes better recycling practices. (23−30) Overall, nonaqueous reprocessing offers a more sustainable, safer, and efficient solution for the management of nuclear waste. Membranes utilizing ionic liquids offer multiple advantages, including enhanced chemical stability, high ionic conductivity, and the potential for customization for specific applications. (31,32) Compared with traditional organic solvent-based membranes, these membranes are more resistant to degradation under harsh conditions. Moreover, ionic liquids can be specifically designed to possess unique properties, making them adaptable for various separation processes. (33,34) However, there are notable disadvantages such as their high cost, possible toxicity, and the challenges associated with their preparation and scalability. Additionally, the viscosity of ionic liquids may create complications in certain applications, and their long-term stability and environmental impact are areas that require further research.
Though the cation exchange mechanism (vide supra) facilitates metal ion extraction involving ionic liquid-based diluents, it has a major disadvantage of the loss of ionic liquid components via aqueous solubility. In this context, functionalized task-specific ionic liquids (TSILs) have been synthesized and employed with encouraging results. The functionalized ionic liquids reportedly induced the metal ion selectively by reducing the total number of components during their biphasic extractive mass transfer. (35−38) A phosphine oxide-based functionalized ionic liquid has been exploited for the extraction of UO22+. (39) The diglycolamide and CMPO-functionalized ionic liquid has been employed for the highly efficient extraction of trivalent lanthanides/actinides like Am3+/Eu3+. (40−43) In a separate study, a malonamide-functionalized ionic liquid has been employed for the extraction of uranyl ions and rare earth elements from lamp phosphor. (44−46) The crown ether-functionalized ionic liquids have also exhibited highly efficient and selective separation of lithium ions. (47) Several attempts were also made to include the functional group into the anionic part of the ionic liquid or as constituent anions. (48−50) In the case of β diketonate-functionalized ionic liquids, mainly with a TTA moiety, a similar highly efficient extraction of trivalent lanthanides has been evidenced...
Here is the structure of the ionic liquids being discussed:
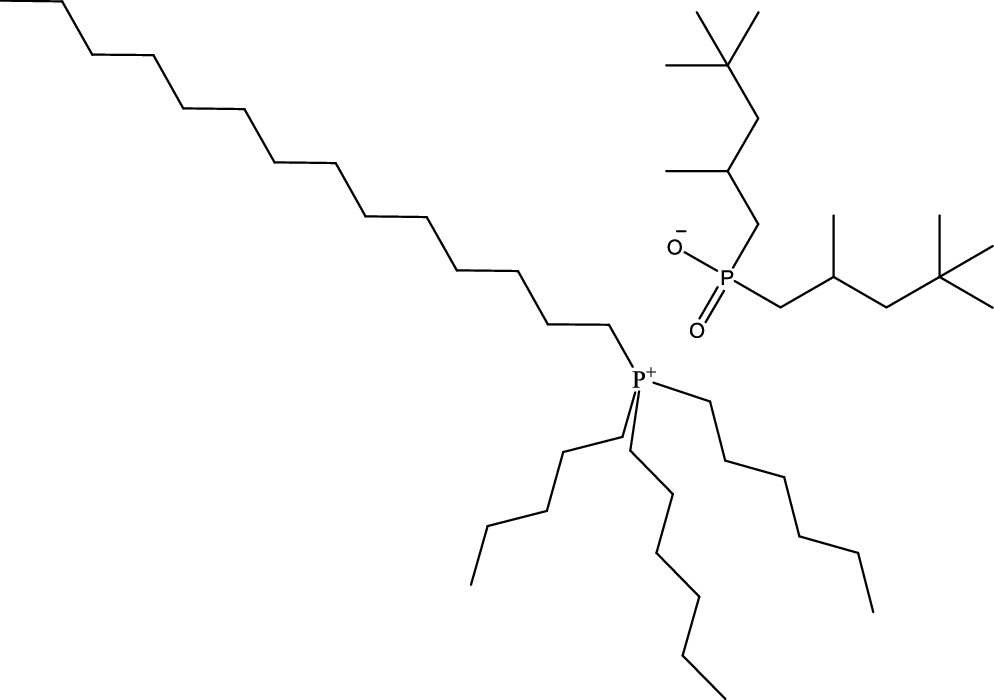
The caption:
Figure 2. Structural formula of trihexyl tetradecyl phosphonium bis (2,4,4) trimethylpentylphosphinate (Cyphos IL 104).
Some distribution coefficients - note the logarithmic ordinate - showing the separation from uranium and americium are shown in the next figure. Americium separations are only necessary in cases where the nuclear fuel contains reactor grade, rather than weapons grade plutonium. (I have been thinking a lot about americium lately - a potentially very cool reactor fuel is certain physics issues can be addressed.)
The figure:
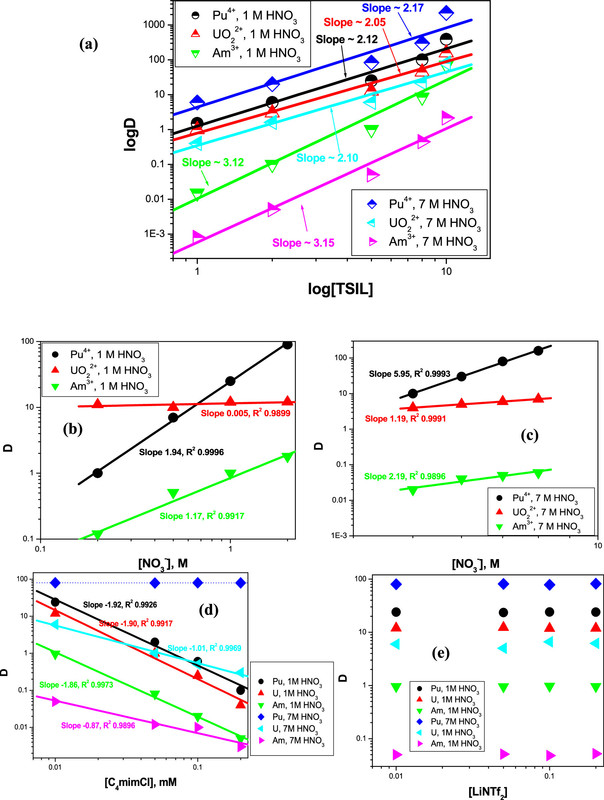
The caption (modified to address the limitations of the DU editor):
The ionic liquid was evaluated for recyclability, with only slight losses in the distribution coefficients.
Similarly, in irradiation experiments it was shown to have reasonable stability with respect to radiation doses up to 1000 Gray.
The system depends upon nitric acid dissolution, which for various reason, I personally find less than desirable. I'm a fluoride volatility kind of guy, but nonetheless the paper is interesting. Regrettably I will not have time to discuss it on a deeper level.
Have a nice day tomorrow.