Environment & Energy
In reply to the discussion: My Son's Other Christmas Present For Me: Rocks to Save the World. [View all]NNadir
(37,277 posts)He's in town for the holidays. The topic involved a certain kind of passive reactivity control using fissiogenic molten CsI which has a fairly high neutron capture cross section for the two I and two of the Cs isotopes, but can be, in effect, a burnable poison. The product of neutron absorption in 127I and 129I would be 130Xe, and 128Xe. 129Xe, as you note, is magnetically active in NMR, and it is a shame that the half-life of 129I is so long, since it would be a source of this nucleon. It has been used in a medical setting in MRI for lung diseases, but I think it's very expensive.
As for burnable poisons, my son tolerates my ideas. He may appreciate them more when I'm dead. (I hope so.)
There has been a number of discussions of recovering xenon from used nuclear fuel; the key being separating it from fission product krypton which includes radioactive 85Kr (t1/2 = 10.76 y).
In fact, some time ago, I attended a lecture on the topic at Princeton University, which was surprising, because in general, Princeton University is an intellectual anti-nuke hellhole except for dreams of fusion, fusion obviously having been far too late to do a damned thing about extreme global heating now being observed. At Princeton University, they want to wait, like Godot, for the so called "renewable energy" nirvana that is not here, did not come, and won't come.
The lecture was by this guy: Moises A. Carreon
I was surprised as hell that there was actually a discussion at Princeton on used nuclear fuel reprocessing. Maybe it was an attempt to be "fair and balanced;" I don't know.
A paper reflecting the topic he discussed is this one:
Microporous Crystalline Membranes for Kr/Xe Separation: Comparison Between AlPO-18, SAPO-34, and ZIF-8 Ting Wu, Jolie Lucero, Zhaowang Zong, Sameh K. Elsaidi, Praveen K. Thallapally, and Moises A. Carreon ACS Applied Nano Materials 2018 1 (1), 463-470.
85Kr could be made to make a photoelectric battery in theory, since ionized Kr (like Xe) has the property of producing a glow very much like daylight, and in fact, is used as such in some lighting applications; I believe some expensive headlights on cars use this approach. There will never be enough 85Kr to make this a major energy source, because of secular equilibrium, but one can imagine specialized areas in which it might have application. Interestingly, the decay product would be the only source of nonradioactive rubidium, 85Rb, since natural rubidium, like natural potassium, is radioactive owing to the very long lived 87Rb isotope that is used in radioactive dating of very old rocks.
It appears, that in the ocean, where 222Rn is formed from the 4.5 billion tons of uranium contained therein, a disequilibrium exists because of the low solubility of thorium in seawater, as well as outgassing of 222Rn, apparently unrestrained by clathrate formation.
This topic is covered in a wonderful book I obtained through the interlibrary loan program at my public library, and scanned in a searchable PDF at Princeton, antinuke hellhole, in defiance of their stated policies of waiting for Godot.
U-Th Series Nuclides in Aquatic Systems
Apparently clathrates may not apply quite as well to radon as to xenon, since the ocean outgases radon as does groundwater.
From the text:
Groundwater can be another source of'222Rn to the atmosphere. Groundwaters typically have 222Rn activities in the range of a few hundreds to a few thousands of dpm/L (Porcelli, this volume). Use of groundwater for agricultural, domestic and industrial purposes would release 222Rn from them to the atmosphere. The flux of Rn from this source could have significant spatial variability and its importance relative to diffusion from soils would depend on the magnitude of groundwater usage. Hussain and Krishnaswami (1980) estimated that in Ahmedabad, a major urban area of India, the flux of 222Rn associated with groundwater usage is a few percent of the soil-derived flux.
cf. ibid, Chapter 2, Church and Sarin, U and Th Series Nuclides in the Atmosphere: Supply Exchange, Scavenging and Applications to Aquatic Processes, Page 14
Figure 2:
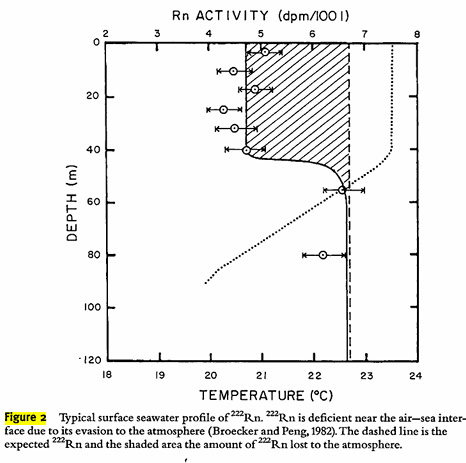
This may be a function of temperature. I have not looked into Rn clathrates to be honest.
Thanks for your comment.