NNadir
NNadir's JournalFour indicted in Sweetwater wind turbine blade dumping probe; cleanup not yet started
Four indicted in Sweetwater wind turbine blade dumping probe; cleanup not yet started
(To get a scale of these things, it is useful to compare the size of the cars in the parking lot.)
RELATED | Texas environmental agency cracks down on wind turbine blade disposal in Sweetwater
The City of Sweetwater, the Sweetwater Police Department, Sweetwater Economic Development and the Nolan County District Attorney’s Office held a press conference Thursday, to provide an update on the status of the wind blades at the location, outline actions taken and discuss the indictments...
...Nolan County District Attorney Ricky Thompson said cases were presented Tuesday to a Nolan County grand jury based on the Sweetwater Police Department investigation. Thompson said four cases were presented, “actually eight cases total,” and said the indictments involve illegal dumping and theft of property with engaging in organized criminal activity. Thompson said the theft-related charge was a first-degree felony and said the illegal dumping charge in Texas “only carries a state jail felony.”
Thompson also said the individuals responsible do not live in Nolan County and “don't even live here in the state of Texas.”
The issue dates back to at least 2022, when two massive piles of old wind turbine blades were reported being stored in Sweetwater and Nolan County. Global Fiberglass Solutions has two storage locations tied to the matter, one inside the Sweetwater city limits and another south of town...
I should expect some "whataboutism" connected with the Texas oil and gas industry, which I acknowledge, but the fact that oil and gas are dirty fuels - albeit necessary in Texas to address the unreliability of the wind - does not make wind energy "clean." Neither wind nor solar nor dangerous fossil fuels are as clean as nuclear energy.)
Note that these blades have been decommissioned after they finished spewing microplastics from their coatings into the air.
(For just one example of the microplastic pollution associated with wind turbines see: Stefania Piarulli et al 2024 J. Phys.: Conf. Ser. 2875 012050 Assessment of microplastics in the sediments around Hywind Scotland Offshore Wind Farm
Based on Danish data, it can be shown that the lifetime of a wind turbine is, on average, less than 20 years, although some wind turbines can function for longer periods (at reduced efficiency) and many are finished within 5 years.
Ercot, the Texas grid, is one of the leading wind producing grids in the country.
According to the Electricity Map, over the last year, the carbon dioxide intensity of the Ercot grid was 288 grams CO2/kWh which, in "percent talk" is 993% higher than that of France, 29 CO2/kWh. Again, in "percent talk," about 8.5% of Texas electricity comes from nuclear energy.
Have a nice evening.
Generational Toxicity and Incomplete Recovery from Spent Lithium-Ion Battery Leachate in an Aquatic Microinvertebrate
The paper to which I'll refer in this post is this one: Generational Toxicity and Incomplete Recovery from Spent Lithium-Ion Battery Leachate in an Aquatic Microinvertebrate Brachionus asplanchnoidis Yi-fan Feng, Yi-fu Xing, and Jia-xin Yang Environmental Science & Technology 2026 60 (4), 2964-2975
One of the big lies in environmental thinking is that batteries in particular, and energy storage in general, are "green." Over the years here I've suffered quite a bit of exposure to this pixilated notion, coupled with the very depressing realization that even with a planet in flames, few people trouble themselves to think about the laws of thermodynamics, a key concept in making decisions about energy, and thus the environment.
This handwaving exercise in mindless credulity is based on the similar lie that so called "renewable energy" is "green, although there is zero evidence that throwing trillions of dollars at it has addressed anything involved with the collapse of the planetary atmosphere.
And, while there is endless fascination with tracing the genetic effects of the big bogeymen at Chernobyl and Fukushima, in the former case concerning the multiple species that have come to thrive in the Viridian exclusion zone in the absence of humans, there is little focus on the mutagenic effects of the industrial products that exist all over the world beyond the exclusion zone.
Well, it would seem that there is some attention being paid, if one bothers to read the paper cited at the outset of this post.
To wit from the introduction:
Field measurements at a landfill containing LIBs found that 42.50% of total Li and 11.45% of total Mn were present in solution, whereas less than 4% of Co, Ni, Al, Cu, and Fe were detected. (11) Lithium cobalt oxide (LCO, LixCoO2) and lithium nickel cobalt manganese oxide (NMC, LixNiyzCo1–y–zO2, 0 less than x, y, z less than 1) nanoparticles exert toxic effects on Daphnia magna in ways not fully explained by dissolved metal levels alone, implicating both ion release and nanoparticle uptake/adherence as key routes. (12) Recent single-cell work with LCO nanoparticles further supports a “two-hit” mechanism: intact nanoparticles are first internalized and act as potent sources of reactive oxygen species (ROS) that activate oxidative-stress response genes, whereas Li+ and CO2+ released from the particles subsequently suppress transcription of these same genes, weakening cellular defenses against ROS. (13) Together, these lines of evidence underscore the need for a mixture-level risk assessment that reflects real spent LIB leachate rather than isolated constituents.
As primary consumers that bridge phytoplankton to higher organisms and accumulate contaminants from lower levels, rotifers facilitate pollutant transfer through the food web; (14−17) Brachionus asplanchnoidis, a coastal planktonic member of the Brachionus plicatilis species complex, therefore provides a model for ecotoxicological assessment at the base of the zooplankton community. (18) They also offer clear experimental advantages─simple body plan, small size, eutely (a fixed number of somatic cells), rapid reproduction, short generation time, clonal propagation, and ease of culture─supporting reproducible ecotoxicology tests. (19,20) Many LIB material recovery facilities (MRFs), owing to convenient logistics and lower transportation costs, are located in coastal areas, and their potential impact zones overlap with the distribution range of marine and estuarine rotifers. (5,21) This spatial coincidence creates a plausible pathway for spent LIB leachate to impact coastal aquatic ecosystems...
There's a lot of nice descriptions of the experimental procedures, which I'll skip, to offer some results:
...and...
...and...
I'll skip sharing the figures in the paper. The text is clear enough.
Don't worry, be happy. There isn't enough cobalt on the planet to cover a month long episode of Dunkleflaute in Germany, as I pointed out some years ago:
The Number of Tesla Powerwalls Required That Would Address the Current German Dunkleflaute Event.
The "batteries will save us" fantasy will come crashing down sooner or later, albeit not without tremendous environmental cost.
The moral cost is delineated in a very recent book I'm going through, to which I've referred in this space, the title of which is a witty, if depressing pun.
From a recent comment I made elsewhere:
The Elements of Power
Subtitle:
...By Nicolas Niarchos
I'm reading it right now, although I have long understood that so called "renewable energy" is not renewable, nor has it ever been about displacing fossil fuels, on which it depends. It was always about attacking the last best chance humanity had at eliminating fossil fuels, nuclear energy.
Have a nice evening.
We have an Iranian scientist in our lab.
He's a fine scientist and a critical partner in our work. I consider him a friend.
(He despises the Iranian government just as much as I despise the orange ignorant pedophile, maybe more.)
He's not here today to work. I don't know where he is. I'm hoping he hasn't been picked up by the American answer to the Gestapo.
Update: I reached him. He's OK. He doesn't know the status of his family. He's OK..
I have a big sigh of relief. We're in the middle of something quite important.
My wife likes to be prepared for everything. Now she wants to prepare with a restaurant survey.
She's really great that way. She always had new clothes when the kids were growing up, clothes she'd bought on sale when they were not big enough to wear them, size 13 shoes for my rapidly growing tall son when he was still a size eleven, toilet paper, paper towels, snow shovels, etc.
We have enough pasta stored to last for a year; rice as well; peanut butter, jelly, flour, you name it. (One needs to be prepared if one's country is taken over by a puerile orange pedophile.)
This weekend she suggested that we survey restaurants in town, Italian of course, so that when one of dies - I get to die first I hope, since it would be harder for me to live without her than for her to live without me - we know what restaurant to which we can take the funeral guests.
I mean, it's a very practical consideration, I have to admit. We aren't spring chickens, so we need to know where to get chicken cacciatore, especially when the family is liberated from my refusal to eat chicken.
She's always ahead of the game. It's one of the many reasons I love her.
Everyone killed in this war is dying because of Epstein. This needs to be repeated often.
Questions asked by 13 and 14 year old kids at a science lecture yesterday.
I went to this lecture yesterday:
Science On Saturday: How Diet and Obesity Shape Our Immune System’s Ability to Kill Cancer
There were 13 and 14 year old kids asking questions that blew my mind, for instance, asking to explain the differences in lysozymes and granulocytes in NK killer cells and how their distribution in cytoplasm was affected by obesity.
I can't remember all the questions kids asked, but I was impressed.
It is a terrible thing, with these fine young minds, we are destroying science in this country.
The lecture should be available as a recording on line in a few weeks.
It was rather amazing, I have to say. Dr. Lynch when asked what teacher inspired her, said her teacher was a nun in Catholic High School who taught evolution, albeit claiming it was inspired by God.
This got a hearty laugh from the audience.
Dr. Lynch is an immigrant, from Ireland.
In former times, the world sent their best and brightest to the United States. No more....
Oceanic DDT Off Los Angeles County from 20th Century Chemical Waste Practices.
The paper to which I'll refer in this post is this one: Identification of DDT+ in Deep Ocean Sediment and Biota in the Southern California Bight Margaret E. Stack, William H. Richardot, Raymmah Garcia, Tran Nguyen, C. Anela Choy, Paul R. Jensen, Johanna Gutleben, Nathan G. Dodder, Lihini I. Aluwihare, and Eunha Hoh Environmental Science & Technology Letters 2024 11 (5), 479-484.
It refers to chemical waste dumping off the coast of Palos Verdes, a wealthy area of Los Angeles County, through which I used to bicycle in my youth from my home in Hermosa Beach to my part time job in Harbor City, (the long route.) It is the "hump" on the corner of Los Angeles County that one can see if one flies into the city and looks South.
The paper is open sourced and is free to read by the public:
Offshore dumping of various chemical waste products was a legal practice in the 1900s, and there are 14 known deep ocean disposal sites off the coast of southern California. (2,4,5) One disposal site, Dumpsite 2, is in the San Pedro Channel between Long Beach, CA, and Santa Catalina Island, CA. The 1985 report provides evidence that DDT waste was disposed at Dumpsite 2, but it was often illegally short-dumped before vessels reached the designated dumpsite. (4,6) Venkatesan et al. further reported in 1996 that the ratio of DDT congeners found at the offshore sites did not match those in either the wastewater discharged onto the PVS or the DDT technical mixture. (7) These early findings were corroborated more recently when waste barrels were imaged by underwater vehicles at locations matching the offshore dumpsites identified by Chartrand et al. in 1985. (4,6,8) However, concurrent investigations by the EPA suggested that DDT manufacturing waste may have been bulk-dumped (i.e., not containerized) near the dumpsites rather than disposed in barrels. (9−11) Together, these studies point to a secondary offshore DDT waste source that has been largely unaccounted for in regional environmental surveys, even when examining biota collected in deep waters. (11)
Evidence of potentially significant offshore DDT waste dumping increases the uncertainty in past estimates of the total magnitude of DDT pollution in the SCB. The DDT pollution on the PVS is well-characterized, but there is a need to further investigate the role of offshore deep dumpsites as a source of DDT to the SCB food web. (2,3,6) Additionally, most DDT surveys examine four to eight typical compounds (DDX), such as p,p′- and o,p′-DDT, DDE, and DDD. However, recent work indicates that marine mammals inhabiting the SCB are exposed to more than 45 DDT-related contaminants. (1) This larger suite of DDT-related chemicals is known as DDT+. (2) DDT+ includes not only DDX but also further degradation products, relatively unknown compounds such as tris(4-chlorophenyl)methane (TCPM), tris(4-chlorophenyl)methanol (TCPMOH), and their isomers and congeners as impurities of DDT technical product, as well as additional DDT-related compounds. (1,2,13) To our knowledge, only Kivenson et al. in 2019 performed a nontargeted analysis to identify DDT+ and other contaminants present in Dumpsite 2 sediments at different locations than in the present study. (6) Their results showed high variability in sediment DDT+ concentrations across two sediment samples (2–4 and 4–6 cm sediment depth), indicating that dumping was nonuniform. Given the small sample size as well the variability, further investigation of DDT+ profiles in deep ocean sediments are warranted. Additionally, while deep ocean sediments have sparse contaminant data, there are no reports on DDT+ in deep sea biota collected from this location. (12)
Our study aims to investigate the halogenated organic compound (HOC) profile of the deep ocean disposal site (Dumpsite 2) using a nontargeted analysis based on comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GC×GC/TOF-MS) and custom mass spectral libraries developed from SCB marine mammal surveys. (1,13−20) Here we (1) determine HOC profiles for sediments collected at Dumpsite 2, with a focus on assessing the occurrence of the 45 DDT+ compounds previously identified in regional bottlenose dolphins (Tursiops truncatus), (1) and (2) assess the potential for DDT+ bioaccumulation in the deep ocean food web by determining the chemical profiles in one invertebrate and three fish species collected from throughout the water column. The results provide foundational knowledge regarding the type and relative abundance of DDT+ waste in Dumpsite 2 sediments and are the first investigation of DDT+ compounds in deep ocean biota...
A figure from the paper shows a map and the contaminated disposal zones:
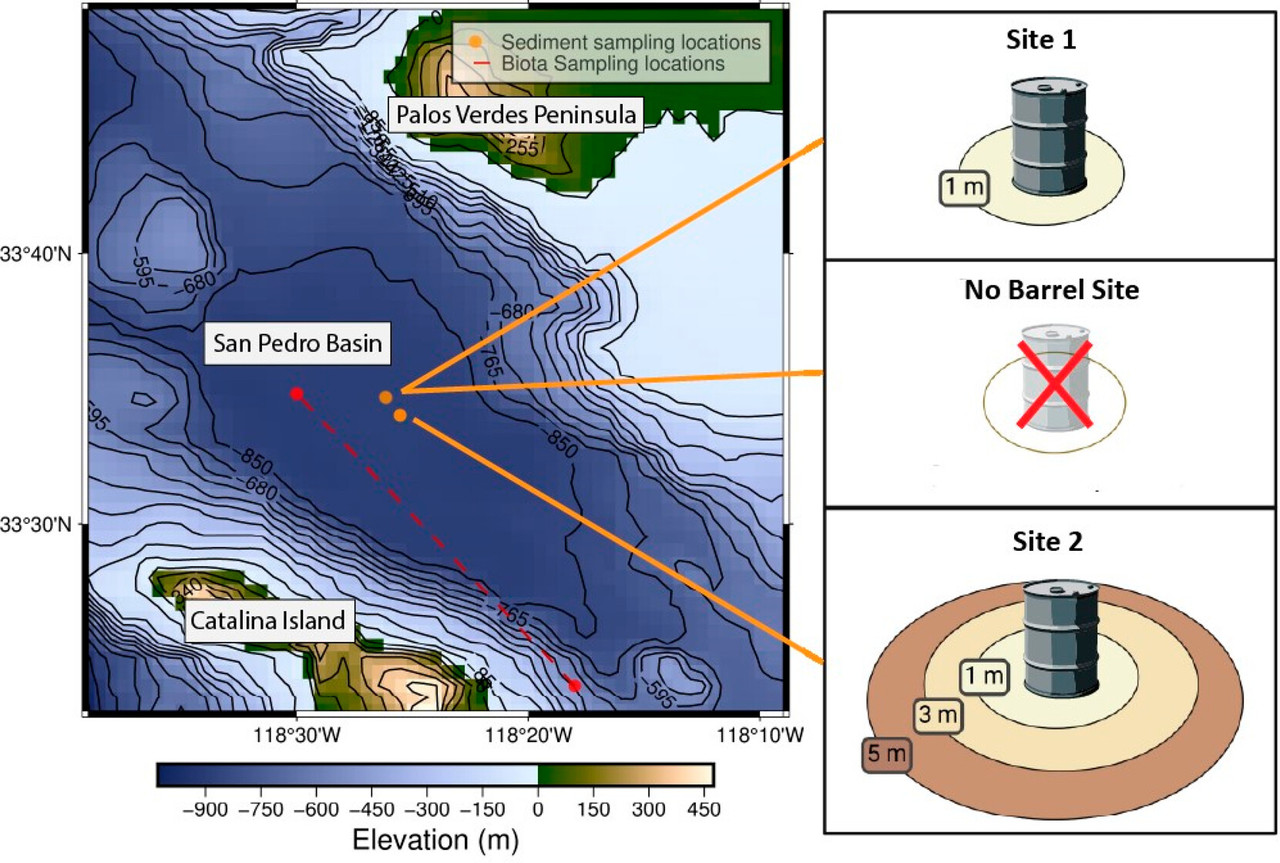
The caption:
Have a nice weekend.
Flood Control Monitoring, Homeless People, and Covid Virus Mutant Spike Protein.
The paper to which I'll refer in this post is this one: Environmental Surveillance of Flood Control Infrastructure Impacted by Unsheltered Individuals Leads to the Detection of SARS-CoV-2 and Novel Mutations in the Spike Gene Anthony Harrington, Van Vo, Michael A. Moshi, Ching-Lan Chang, Hayley Baker, Nabih Ghani, Jose Yani Itorralba, Katerina Papp, Daniel Gerrity, Duane Moser, and Edwin C. Oh Environmental Science & Technology Letters 2024 11 (5), 410-417.
The paper, which is almost two years old, is open access and is free to read by the general public.
For those who may not remember, the spike protein is the target of most vaccines, and thus a mutation is potentially a serious matter.
Despite being free to read, I'll briefly excerpt it:
The introduction:
In addition to reporting on SARS-CoV-2 viral RNA in wastewater, several studies have described the detection of viral material and human fecal contamination in flood control infrastructure due to overflowing sanitary sewers, sewer collection system leaks, and direct human inputs. (16−18) In cities like Las Vegas, environmental factors (e.g., relatively mild winters) coupled with city ordinances criminalizing unsheltered homelessness have led to the establishment of encampments in remote areas, including flood control channels, where makeshift toilets and open defecation are common. (18) These unsheltered individuals are at higher risk of infection, morbidity, and disease-associated mortality because of their limited access to health care services. (19−21) Thus, wastewater surveillance of homeless shelters (8) and environmental surveillance of flood control infrastructure may help characterize public health conditions and disease transmission, including for COVID-19, within this particularly vulnerable population that is often underrepresented in traditional clinical surveillance data.
SARS-CoV-2 viral RNA can persist for up to several weeks in environmental waters, and potentially longer depending on the temperature of the water. (22) In addition, SARS-CoV-2 infected individuals shed maximum viral RNA in fecal matter at the onset of infection and up to seven months after the initial infection. (8,22−24) While previous studies on SARS-CoV-2 in surface waters, including stormwater, have documented SARS-CoV-2 RNA levels by quantitative polymerase chain reaction (qPCR), (16,17) whole genome sequencing (WGS) of samples from flood control channels (e.g., urban runoff and stormwater) to generate SARS-CoV-2 variant information is less common. (16,17,25) This could be due to challenges with collection and analysis of these complex samples, specifically performing WGS on low viral loads or in the presence of unique PCR inhibitors which negatively influence the success of sequencing workflows. (7,26) Obtaining knowledge about the SARS-CoV-2 variant composition in urban runoff and stormwater could support public health surveillance of new reservoirs of viral variants. Therefore, the objectives of this study were to (1) determine if SARS-CoV-2 RNA could be detected in environmental water samples collected from flood control infrastructure that is known to be impacted by unsheltered individuals, (2) conduct amplicon-based WGS of SARS-CoV-2 from these environmental water samples, (3) compare SARS-CoV-2 variants present in the environmental water samples with those circulating in the local community (via wastewater and clinical surveillance), and (4) investigate if novel mutations could be detected...
Again, the paper is open and free to read.
We may never know how many homeless people died from Covid, the first major health disaster overseen by the orange pedophile in the White House, which he is trashing. The second, and possibly the worst health disaster for which this awful excuse for a human being is responsible is the elevation of brain worm boy Bobby to oversee our Department of Health.
In Lieu: No Prisoner Be.
A friend called me up this morning and said he had two free tickets to "The Opera" at a local theater, which turned out to be, after some confusion, the Richardson Auditorium at Princeton University.
He told me that he had no use for opera, that he'd rather "be stabbed in the eye" than go to one, his exact words.
So I called my wife, with whom I fell in love 42 years ago, because she was open to anything in culture - one of the first places I took her when our friendship was developing was to a Nonesuch Records benefit featuring John Cage reading the poems of Marcel Duchamp - and, just as she had when we were young, she said she'd love to go, because it was something we'd never done, and so we went.
The "Opera" turned out to be a live performance of Emily, No Prisoner Be, the poems of Emily Dickenson put to music and sung in the powerful mezzo soprano voice of Joyce Didonato accompanied by the string trio Time for Three with music written and scored by Kevin Puts, played in alternate fits of atonality mixed with fire and bee like breath, violin and violin viola and bass.
Thus spake Emily:
Because I could not stop for Death –
Because I could not stop for Death –
He kindly stopped for me –
The Carriage held but just Ourselves –
And Immortality.
We slowly drove – He knew no haste
And I had put away
My labor and my leisure too,
For His Civility –
We passed the School, where Children strove
At Recess – in the Ring –
We passed the Fields of Gazing Grain –
We passed the Setting Sun –
Or rather – He passed Us –
The Dews drew quivering and Chill –
For only Gossamer, my Gown –
My Tippet – only Tulle –
We paused before a House that seemed
A Swelling of the Ground –
The Roof was scarcely visible –
The Cornice – in the Ground –
Since then – 'tis Centuries – and yet
Feels shorter than the Day
I first surmised the Horses' Heads
Were toward Eternity –
It was a beautiful evening, in the cold night, wrapped in the hedgerows of broken ploughed snow.
I felt my love at 20 again, and I at 31, her child, lost as if returned to Dewey Redman signing in Keith Jarrets Birth
Somewhere off, I knew some doddering pedophilic fool muttered incoherently something made of nothing and and certainly nothing here
Quoth Emily, Sung through Joyce DiDonato's powerful voice:
No Prisoner be—
Where Liberty—
Himself—abide with Thee—
No Prisoner be—
Where Liberty—
Himself—abide with Thee—
No Prisoner be—
Where Liberty—
Himself—abide with Thee—
It was perfection in an vastly imperfect life, and so the with that imperfect rotting organ somewhere off, liberty, himself abided with me.
Ms DiDonato led the sold out house to sing that:
No Prisoner Be-
Where Liberty-
Himself -abide with Thee-
When it was over, I realized I was weeping.
The Good News About This Major Storm.
From the Drought Monitor:
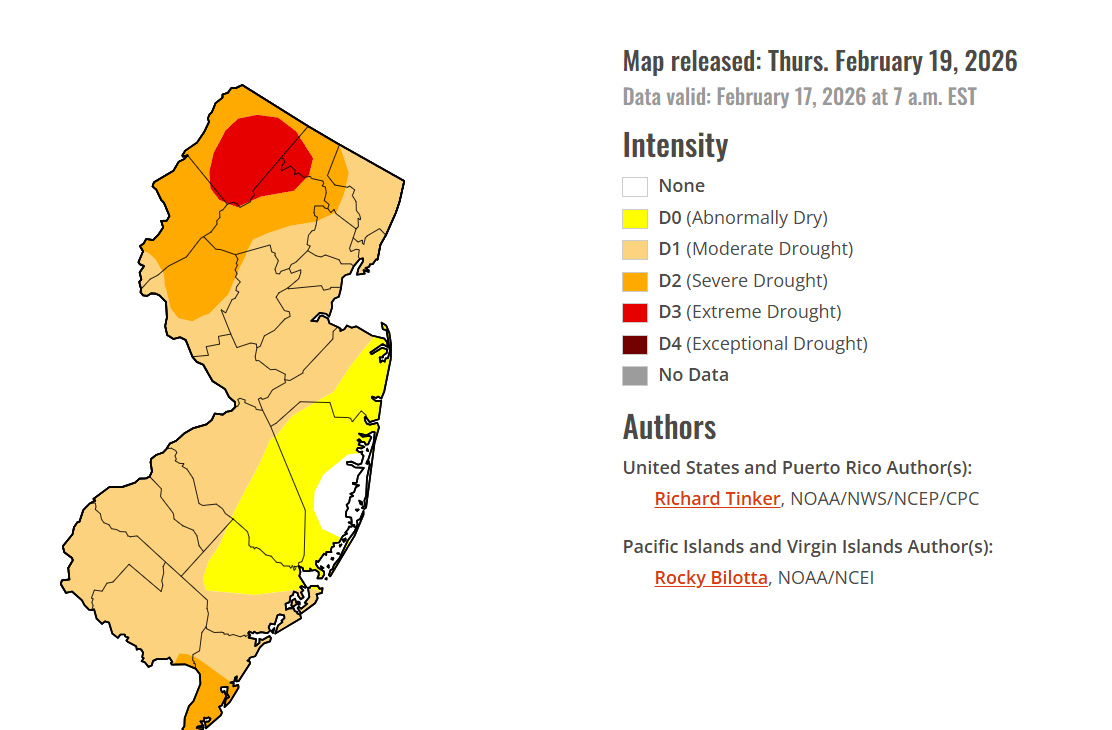
NJ Drought Monitor
Melting snow does better at restoring groundwater than rain.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 37,787